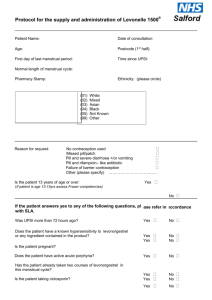
relative contra-indications
advertisement

THE <NAME> PRACTICE PROTOCOL Title: Depo-Provera 150 mg(ml) DMPA Protocol Review Date: March 2014 Version: 1.3 ABSOLUTE CONTRA-INDICATIONS Pregnancy. History of arterial thrombosis. Liver disease. Women who wish to conceive soon after using injection. Unexplained vaginal bleeding. RELATIVE CONTRA-INDICATIONS Risk factors for arterial disease. Significant risk factors for osteoporosis: corticosteroid treatment, anorexia nervosa, bulimia, or over 45. NEW ADVICE FOR PRESCRIBERS: The CSM advises that: In adolescents, Depo-Provera may be used as first-line contraception but only after other methods have been discussed with the patient and considered to be unsuitable or unacceptable. In women of all ages, careful re-evaluation of the risks and benefits of treatment should be carried out in those who wish to continue use for more than 2 years. In women with significant lifestyle and/or medical risk factors for osteoporosis, other methods of contraception should be considered. STARTING ROUTINES First injection should be given before day 5 of the cycle. Contraceptive effect is then immediate. Careful POP or COC uses – at any time. Postpartum – 5/6 weeks after delivery. (Lactation not affected). Post miscarriage or TOP – within 7 days and immediate contraceptive effect. INITIAL COUNSELLING Discuss: Possible amenorrhoea or frequent irregular bleeding (especially for first 12 weeks). Possible weight gain. Delay in return to fertility (9-12 months). Theoretically long-term risk of arterial disease and osteoporosis. Mention that the risks and benefits of treatment need to be re-evaluated after 2 years use. Give information leaflet. Inform can telephone for advice should any anxieties arise. MANAGEMENT AND ADMINISTRATION Initial consultation check BP and weight. Thereafter ensure BP and weight recorded at least yearly. Shake vial and give IM in buttock (do not rub injection site). Record menstrual pattern/amenorrhoea. Print script for sending to Upton. Plan date for next dose – advise better to be a week or two early than late. (WHO advise that repeat injections can be given up to 2 weeks early). If late do not have SI. Page 1 of 2 Depo-Provera Guidelines Protocol v1.3.doc Author: Dr F Ballinger/Lyn Fitzsimmons Reviewed: 19 December 2012; Created: March 2006 THE <NAME> PRACTICE PROTOCOL OVERDUE INJECTIONS p345 Women attending up to 2 weeks late for repeat injection of DMPS may be given the injection without the need for additional contraceptives. (This is C.E.U guidance followed by FSRH although outside the licensed use.) Patients should be advised to attend for the next injection in 12 weeks not longer. 14 weeks +1 day or more: Injection may be given if no UPSI (abstinence or barrier method). Addition contraception advised for next 7 days. Pregnancy test in 21+ days if barrier method used. 14 weeks + 1 day or more and UPSI in the past 3 days* Offer Levonelle 1500 or a copper IUD. If accepted the injection can be given and additional contraception advised for the next 7 days. A pregnancy test should be checked in 21+ days. 14 weeks + 1 day or more and UPSI in the last 4-5 days* Offer a copper IUD – if accepted and fitted the injection can be given. No additional contraception required. A pregnancy test should be check in 21+ days. 14 weeks + 1 day or more and UPSI more than 5 days ago* Emergency contraception is not indicated. Condoms or abstinence advised for 21+ days. A pregnancy test should then be checked – if negative the injection and additional contraception advised for the next 7 days. * Not applicable if unprotected sex only occurred within 14 weeks of last DMPS injection. REVIEW AFTER 2 YEARS After 2 years of continuous depo-provera use, (or earlier if symptoms of low oestrogen e.g. dry vagina, hot flushes,) women should be reviewed by doctor/family planning trained nurse to reevaluate the risks and benefits of treatment. At 2 years review: 1. Ask her if she wants to change e.g. vasectomy, sterilisation, IUD, POP (no evidence of hypooestrogenism, osteoporosis, or arterial disease in long-term uses of any kind of POP p309), Nexplan (does not inhibit FSH). (Dr Armitage Jan 2004 does not think Implanon is a safer alternative, general FP view presently is that it is). 2. If she wants to continue – discuss diet, exercise and cessation of smoking. 3. Oestradiol and FHS levels are not of value. If risk factors for osteoporosis arrange Dexa scan. (Dr Armitage in Jan 2004 recommends testing oestradiol and if consistently less than 150 add back some oestrogen. However present FP view is not to check oestradiol levels). Refs: Your Questions Answered, Contraception. 2004 4th Ed. John Guillebaud. Churchill Livingstone. Updated Prescribing Advice on the Effect of Depo-Provera contraception on Bones. 18th November 2004, Committee on Safety of Medicines. FSRH Guidance 2008 (Faculty of Sexual and Reproductive Healthcare Clinical Effectiveness Unit in Collaboration with the Royal College of Obstetricians and Gynaecologists). Page 2 of 2 Depo-Provera Guidelines Protocol v1.3.doc Author: Dr F Ballinger/Lyn Fitzsimmons Reviewed: 19 December 2012; Created: March 2006