Acoustic trauma R3吳俊璟
advertisement
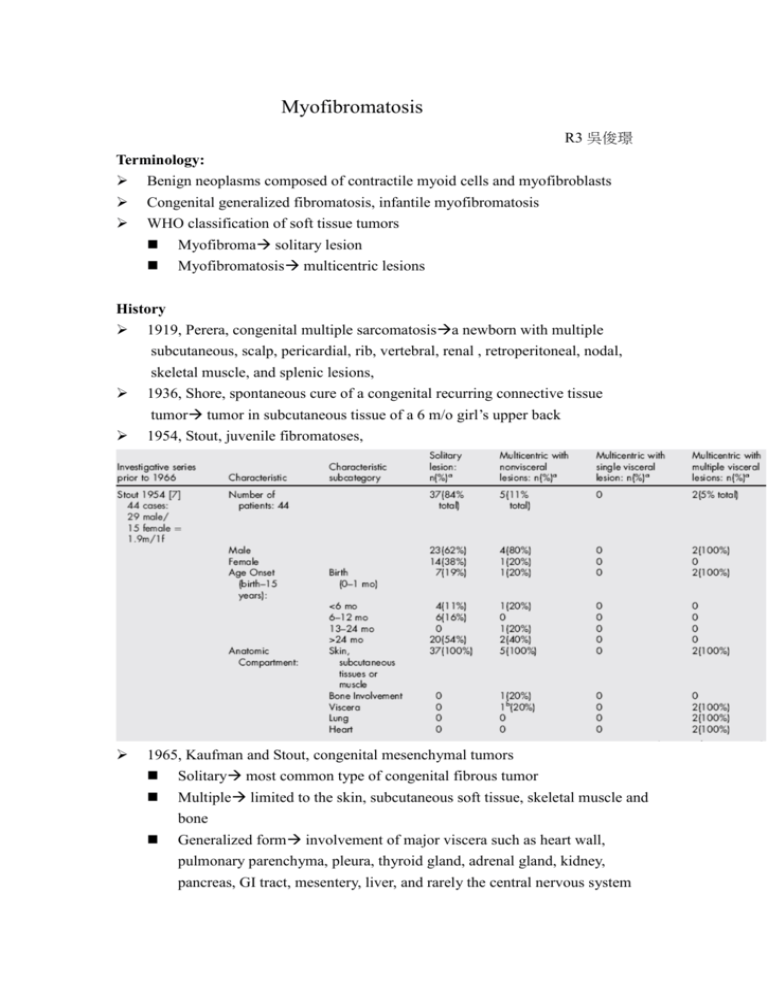
Myofibromatosis R3 吳俊璟 Terminology: Benign neoplasms composed of contractile myoid cells and myofibroblasts Congenital generalized fibromatosis, infantile myofibromatosis WHO classification of soft tissue tumors Myofibroma solitary lesion Myofibromatosis multicentric lesions History 1919, Perera, congenital multiple sarcomatosisa newborn with multiple subcutaneous, scalp, pericardial, rib, vertebral, renal , retroperitoneal, nodal, skeletal muscle, and splenic lesions, 1936, Shore, spontaneous cure of a congenital recurring connective tissue tumor tumor in subcutaneous tissue of a 6 m/o girl’s upper back 1954, Stout, juvenile fibromatoses, 1965, Kaufman and Stout, congenital mesenchymal tumors Solitary most common type of congenital fibrous tumor Multiple limited to the skin, subcutaneous soft tissue, skeletal muscle and bone Generalized form involvement of major viscera such as heart wall, pulmonary parenchyma, pleura, thyroid gland, adrenal gland, kidney, pancreas, GI tract, mesentery, liver, and rarely the central nervous system 1981, Chung and Enzinger, AFIP(Armed Forces Institute of Pathology) classification, infantile myofibromatosis myofibroblast: both fibroblast-like and smooth muscle-like feature 2 2002, WHO classification: myofibroma/myofibromatosis 3 Epidemiology From newborn to old age, 90% during the first two years of life Solitary lesions are more common Most common sits: cutaneous/ subcutaneous tissues and skeletal muscles usually of head and neck region Oral cavity tongue/mandible are most common sites Slight male predominance of 1.4 to 1.9 males: 1 female controversial Familial associated case: multicentric type with nonvisceral lesions greater numbers of recurrence, but no increased mortality AD or genetic heterogenecity Myofibromatosis in ear 1992, Hogan and Salassa, 43 y/o woman, recurrent benign in left external ear canal for 3 years, erosion of bony canal wall excision with FTSG reconstruction 1999, Balakrishnan, 50 y/o female, left pinna swelling for 5 months, antibiotic and excisonal biopsy recurrence, wider excision 2004, Izumaru, 38 y/o man, progressive swelling of left external auditory meatus for 3 months excision Histopathologic feature: Multinodular proliferation with a zoned configuration biphasic pattern Periphery: light-stained area composed of short fascicles or whorls of myofibroblast that are spindle shaped, pale pink cytoplasm with elongated, tapering nuclei. Center: dark-stained area, smaller, less differentiated, less cytoplasm and larger basophilic nuclei; thin walled, irregularly branching hemagiopericytoma-like vessels may develop among these cell. 4 Immunohistochemical analysis: positive strong reaction to vimentin, actin and α-SMA, negative reaction to desmin, S-100 and cytokeratin Necrosis and calcifications in tumors in the whole body, but rare in oral cavityspontaneous regression: most in children Etiologic cause Remains unknown Trauma? Estrogen? Basic fibroblast growth factor(bFGF): a fibroblast mitogen, promote angiogenesis and lesion growth in myofibromatosis, elevated in the acute phase of disease Cytogenetic status: deletion of the long arm of chromosome 6; abnormal chromosome 9, 16 no signature recurring chromosomal or other molecular marker unique to myofibromatosis has been established Differential diagnosis Neurogenic tumor: immunoreactivity for S-100 Leiomyoma: immunoreactivity to desmin and h-caldesmon Low grade myofibroblastic sarcoma: adult, infiltrative growth pattern with nuclear atypia Nodular fasciitis: mucin-rich stroma, extravasated red blood and lymphocytic inflammation Desmoid-type fibromatosis: more aggressive and destructive behavior, strong immunoreactivity for beta-caenin, lack bi-phasic pattern Fibrous histiocytoma: xanthoma cells, touton-type giant cells, associated with a lymphocytic infiltration, positive immunorectivity to CD68 Hemangiopericytoma: immunoreactivity to CD34 and CD99 5 Infantile/ congenial fibrosarcoma: mitotic activity, invasive borders, greater cellularity, chromosomal translocation Clinical presentation Skin colored or reddish to purple dome-shape nodules, form a few millimeters to several centimeters. In oral cavity: rubbery-to firm painless swelling to the affected area, superficial and bulged or in the deeper subcutaneous area Duration: 2 weeks to6 months, mean 1~2 months Multicentric type with multiple visceral lesion poor prognosis, especially lung involvement Deaths in myofibromatosis caused by mechanical compression and organ function disruptive effects Image study CT: Iso-attenuating with muscle, well-demarcated isodense mass that absorb contrast Occasionally heterogenous mass with both multicystic and solid areas and occasional areas of bony remodeling, calcification MRI: T1 a mass with a center that has low signal and enhancement after gadolinium T2 hyperintense 6 Treatment 1. Surgical excision Conservative surgical excision is recommended Biopsy is necessary Wide excision if rapid growth or involvement adjacent bony structure 2. Chemotherapy: for myofibromatosis that causing significant morbidity vinblastine and methotrexate, 2 survived, cyclophosphamide tamoxifen, vincistine, actinomycin D and cyclophosphamide dactinomycin, vincristine sulfate, and cyclophosphamide 3. Close follow-up All forms of myofibromatosis may exhibit spontaneous regression 1/2~1/3 Young children suspected of having one or more lesions biopsy, skeletal survey, chest X-ray, ultrasound, CT of the thorax and abdomen 7 References: 1. Vered M, Allon I, Buchner A, Dayan D.Clinico-pathologic correlations of myofibroblastic tumors of the oral cavity. II. Myofibroma and myofibromatosis of the oral soft tissues.J Oral Pathol Med. 2007 May;36(5):304-14. 2. Turner J, Skinner M, Caplan MJ, Gillespie MB.Pathology quiz case 1. Infantile myofibromatosis. Arch Otolaryngol Head Neck Surg. 2007 Jun;133(6):620, 622-3. 3. Matthews MR, Cockerell CJ.An historic perspective of infantile myofibromatosis. Adv Dermatol. 2006;22:279-305. 4. Koujok K, Ruiz RE, Hernandez RJ.Myofibromatosis: imaging characteristics. Pediatr Radiol. 2005 Apr;35(4):374-80. 5. Corson MA, Reed M, Soames JV, Seymour RA.Oral myofibromatosis: an unusual cause of gingival overgrowth.J Clin Periodontol. 2002 Nov;29(11):1048-50. 6. Foss RD, Ellis GL.Myofibromas and myofibromatosis of the oral region: A clinicopathologic analysis of 79 cases.Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 Jan;89(1):57-65. 7. Balakrishnan R, Pujary K, Shah P, Hazarika P.Solitary adult myofibroma of the pinna.J Laryngol Otol. 1999 Feb;113(2):155-7. 8. Beck JC, Devaney KO, Weatherly RA, Koopmann CF Jr, Lesperance MM. Pediatric myofibromatosis of the head and neck. Arch Otolaryngol Head Neck Surg. 1999 Jan;125(1):39-44. 9. Hogan SF, Salassa JR. Recurrent adult myofibromatosis. A case report. Am J Clin Pathol. 1992 Jun;97(6):810-4. 10. Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, Edgar M, Shah JP. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006 May;132(5):517-25. 11. Izumaru S, Yoshida Y, Nakashima T. A solitary fibrous tumor in the external auditory meatus. Auris Nasus Larynx. 2004 Mar;31(1):65-7 12. Soper JR, De Silva M.Infantile myofibromatosis: a radiological review. Pediatr Radiol. 1993;23(3):189-94. 8