IVIg - Long Term Blue-Grey Form 2 v1 Jul15
advertisement

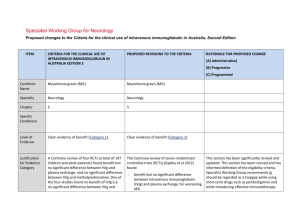
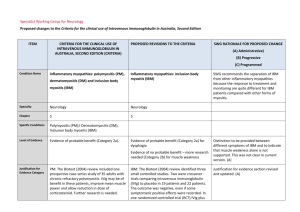
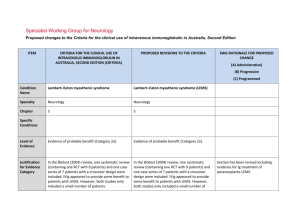
IVIg ASSESSMENT PANEL – Long Term Blue & Grey – FORM 2 This form must be signed by the consultant who will be responsible for the treatment. Please note that the Panel may be unable to reach a decision if inadequate information is provided. Please complete this form and return it to: buc-tr.formularyteam@nhs.net who will then forward it on to Thames Valley IVIG Panel CASE No: DATE FIRST CONSIDERED: PATIENT DETAILS: DATE OF PANEL: SHA/PCT code: NHS NUMBER (essential): NAME: DOB : Height: Weight: GP Name/Address: NB: This will be anonymised before consideration by the Panel 1. Indication for IVIg: (For blue and grey (listed) indications please ensure that your indication matches one of the ‘indications’ listed in the DH Demand Management Clinical Guidelines – 2011 Update, summary tables on p18-25 for blue and table on p26 for grey. Conditions not covered by the guidelines are ‘grey-unlisted’ – please provide a full description of the condition) 2. Selection criteria (ie why do you want to use IVIg in this patient now?): Blue indications: please check the summary tables in the IVIg 2011 update document and: i) list the selection criteria you have used in line with those specified in the table; and ii) give baseline values (ie current, pre-IVIg treatment) for each. Grey indications: please specify relevant selection criteria in line with your understanding of the evidence for IVIg use in this indication, with baseline values Oxford University Hospitals NHS Trust IVIg Panel Form v.4 May 2012 1 3. Previous therapies & responses Was plasma exchage considered [please circle] Not applicable Tried & Failed Considered but not available Considered but patient not suitable Corticosteroids Alternatives tried [please circle] None Cyclophosphamide Methotrexate Ciclosporin Rituximab Other [please state] 4. What is the current therapy & what response has there been? 5. What do you want to achieve with Ig treatment and how will this be monitored (outcomes and efficacy tracking)? Blue indications: list the outcome measure(s) given in the summary tables in the 2011 Update for this condition. For each give a baseline value and the value that you would consider a ‘successful outcome’ from treatment (which will then be used for monitoring and review). Grey indications: list the outcome measure(s) that you expect to achieve based on your interpretation of the evidence. For each give a baseline value and the value that you would consider a ‘successful outcome’ from treatment (which will then be used for monitoring and review). Outcome (efficacy tracking measure) Baseline (pre-Ig) value Value that will constitute ‘successful outcome’ from Ig 6. For grey indications: please attach the evidence supporting clinical efficacy in this indication and demonstrate how it applies to this patient Oxford University Hospitals NHS Trust IVIg Panel Form v.4 May 2012 2 7. What treatment plan do you propose to follow? This must include: (i) Dosage: For blue indications: please state the intended dosage (g/kg and interval) and confirm that this conforms to that given for this indication in the summary table of the 2011 Update document. For grey indications: please state the intended dosage and how this links to evidence of effectiveness. For all indications please confirm that dosage will be based on ideal body weight (calculated as described in the 2011 Update). (ii) How do you intend to confirm ongoing need for Ig? Eg plan to reduce dosage and/or increase dosage interval; occasional use of placebo; trial withdrawal of Ig etc. (iii) How will you withdraw IVIg if the expected outcomes are not met? (iv) Have you made the patient aware of the stopping criteria? (v) Who will manage the ongoing IVIg therapy? 8. Please indicate the anticipated cost of Ig treatment for this patient (total cost for short term; annual cost for ongoing). Please ensure that you will use the Ig product with lowest acquisition cost. PREPARED BY: (Please print: Name, Address and Contact Telephone No) SIGNATURE: ..................................................... DATE ................................... Please return completed form to: TV IVIg Panel c/o Angela Welby, Clinical Unit Manager, Clinical Immunology, Level 4 Academic Street, John Radcliffe Hospital, Oxford. OX3 9DU Oxford University Hospitals NHS Trust IVIg Panel Form v.4 May 2012 3