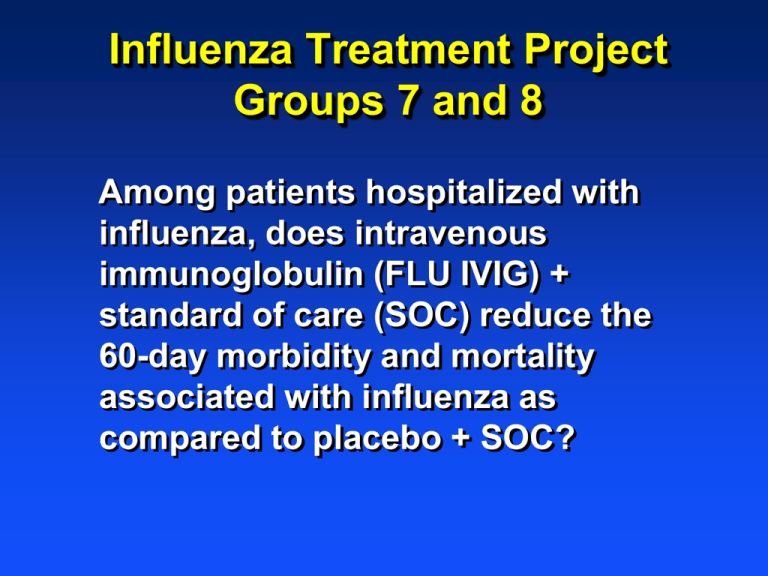
Influenza Treatment Project
Groups 7 and 8
Among patients hospitalized with
influenza, does intravenous
immunoglobulin (FLU IVIG) +
standard of care (SOC) reduce the
60-day morbidity and mortality
associated with influenza as
compared to placebo + SOC?
Background and Rationale
Morbidity and mortality associated with influenza is
substantial – estimated at 500,000 deaths
worldwide.
A recent meta-analysis of observational studies
found that neuraminidase inhibitor treatment was
associated with significant reduction in mortality
among hospitalized patients if treatment was given
within 48 hours of onset.
Many patients are not hospitalized within 48 hours
of symptoms.
Preliminary studies with hyperimmune intravenous
immunoglobulin (IVIG) suggest there may be
benefit associated with their use.
Figure 7. Age distribution of projected global and regional respiratory mortality, for both pandemic
and seasonal influenza mortality estimates.
Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, et al. (2013) Global Mortality Estimates for the 2009 Influenza Pandemic from the GLaMOR
Project: A Modeling Study. PLoS Med 10(11): e1001558. doi:10.1371/journal.pmed.1001558
http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.1001558
From: Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1
Treatment?
Ann Intern Med. 2006;145(8):599-609. doi:10.7326/0003-4819-145-8-200610170-00139
Date of download:
11/29/2013
Copyright © The American College of Physicians.
All rights reserved.
From: Meta-Analysis: Convalescent Blood Products for Spanish Influenza Pneumonia: A Future H5N1
Treatment?
Ann Intern Med. 2006;145(8):599-609. doi:10.7326/0003-4819-145-8-200610170-00139
Date of download:
11/29/2013
Copyright © The American College of Physicians.
All rights reserved.
Influenza IVIG: Recent Studies
• 35 ICU patients at 5 hospitals in Hong Kong with severe A(H1N1)pdm09
infection on standard antiviral treatment requiring ventilatory support:
randomized to receive H-IVIG (17 pts) or normal IVIG (18 pts)
• Overall mortality was similar:
• 5 (29.4%) H-IVIG participants died
• 4 (23.5%) IVIG participants died.
• H-IVIG treatment was associated with significantly lower day 5 (p=0.04)
and 7 (p=0.02) post-treatment viral load compared to IVIG.
• Subgroup analysis:
• ? beneficial effect of H-IVIG on mortality among participants treated
within 5 days of symptom onset (0/12 deaths on H-IVIG versus 4/10
deaths on IVIG)
• Safety: no adverse safety signals of H-IVIG treatment reported.
Influenza IVIG: Unknowns
• Influenza mortality due to ongoing viral replication versus host
inflammation?
• Ability of antibodies to favorably mediate disease pathogenesis?
• Optimal dose of hyperimmune plasma/IVIG?
• Is an HAI titer of ≥ 1:40 sufficient?
• Optimal timing of hyperimmune plasma/IVIG treatment?
• Does a therapeutic window close “x” days after symptom onset?
• Possibility of therapeutic harm?
• Does hyperimmune plasma/IVIG have any role in “complicated” influenza
with secondary infections (e.g. bacterial pneumonia)?
Infusion Procedure - 1
Storage of IVIG and Timing of Infusion
Store
IVIG at -20º C and thaw immediately
before use.
After thawing IVIG, it is added to saline based
on weight to obtain desired 500 mL volume
Begin infusion within 4 hours of preparation
of study treatment and soon as possible after
randomization
Infusion of study treatment must be
completed within 8 hours of preparation
Infusion Procedure - 2
Considerations in blinding
IVIG
has a different color than placebo so
use an sleeve over the infusion bag
Do not shake the infusion bag as IVIG will
bubble
Infusion administration
Infuse
at room temperature over a
minimum of 2 hours
Can
vary administration rate as per local
guidelines
Questions To Consider
Location of sites – both Northern and
Southern Hemisphere would permit
enrollment year round. Need many sites
because it not clear where influenza will
strike.
Feasibility of maintaining double-blind
Primary endpoint definition; definition of
secondary endpoints, including safety.
Inclusion criteria, e.g., duration of
symptoms and confirmation of influenza.