Antiviral agents
advertisement

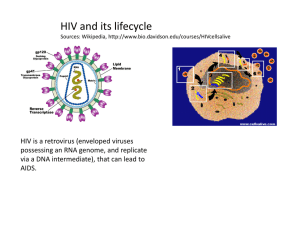
Antiviral agents Viruses are non-cellular, infectious agents which take over a host cell to survive and multiply. Can infect human, plant, animals and bacterial cells. More than 400 different types capable to infect human. Responsible for influenza, chicken pox, measles, mumps, viral pneumonia, rubella and smallpox and many other viral infections. Viruses can be transmitted to human by a variety of ways: Through air by the infected host sneezing or coughing. Through arthropods or ticks such as yellow fever and Colorado tick fever. Through physical contact (for some viruses which can not survive outside the host such as: HIV Cold sore. Genital herpes. Rabies. Food-borne or water borne viruses such as hepatitis A and E and viral gastroenteritis. Viral infections were responsible for the major epidemics worldwide: Smallpox weakened the Roman Empire. Lethal viruses in Africa such as Ebola, Lassa. Sever acute respiratory syndrome (SARS) during 2003 in the Far East. Recently H1N1 The structure of viruses Can be classified as: DNA viruses: contains either single or double strand DNA RNA viruses: contains single strand RNA (ssRNA), but some have double strand RNA. The nucleic acid is protected within a protein coat called capsid. The capsid contains nucleic acid is called Nucleocapsid. The whole structure of virus is called virion (the form that the virus takes when it is outside the host cell) Membrane Nucleic acid Capsid Viral protein RNA polymerase The size of virion can vary from 10nm to 400nm. The outer surface of viral cell Life cycle of virus HIV protease is required to process the transcribed and translated proteins for the new virions Inhibition of RT would prevent conversion of viral RNA genome into DNA for incorporation into the host’s replicatory system Vaccination Is the preferred method of protection against viral diseases. Extremely successful against childhood diseases such as polio, measles, mumps, smallpox and yellow fever. Works by: introducing the body to foreign material having molecular similarity to some component of the virus. Introducing killed or weakened version of the virus. Or administer fragments of virus having the characteristics antigen. Vaccination The body can recognize the molecular fingerprint of the virus and develop specific antibodies against these antigenic structure. Vaccines are currently under investigation for HIV, genital herpes and Ebola virus (causes haemorrhagic fever): These viruses have rapid gene mutation that results in constant changes to the amino acid composition of surface glycoprotein (the surface antigenic component). Patients with weak immune system are not likely to benefit from vaccination. Virus is a hard target Most of the time the virus spend in the host will be inside the host cell. This effectively protect the viral cell from the host immune system as well as from available circulating enzymes. Another problem appears in treating viral infections is the fact that there are limited number of potential drug targets since viruses use the host biochemical mechanisms to multiply. Antiviral agents The first effective antiviral agents appear in 1960s and only three were clinically available: Idoxuridine and vidarabine for herpes infections. Amantadine for influenza A infections. Growing interest in finding effective antiviral agents after that was due to: The need to tackle AIDS spread. The increased understanding of viral genomic sequence and infectious mechanisms. Antiviral agents for DNA viruses Mainly against herpes viruses such as cold sore, genital herpes, chicken pox, eye diseases. Three major mechanisms of actions: 1. Inhibition of viral DNA polymerase. 2. Inhibition of tubulin polymerization. 3. Antisense therapy: which blocks the translation of viral RNA. Acyclovir Acyclovir triphosphate prevents DNA replication By two ways: • Can bind to DNA polymerase instead of deoxyguanosine triphosphate. • Can be incorporated into the growing DNA chain… discontinuation of chain extension due to the absence of 3’ hydroxyl group. Acyclovir selective toxicity Why Acyclovir does not inhibit DNA polymerase in normal, uninfected cells: The first step in phosphorylation requires the viral version of kinase (100 times more active than the host enzyme). There is a selective uptake of acyclovir by infected cells. Acyclovir triphosphate is 50 times more selective on viral DNA polymerase compared to the cellular polymerase. Acyclovir analogues Were synthesized mainly to overcome the poor water solubility and to improve oral availability. Valaciclovir Is an L-valyl ester prodrug of acyclovir. Absorbed more effectively from the gut than acyclovir although it has the same polarity. That fact that the D-valyl prodrug has poor absorption suggesting that there is a special transport system (intestinal oligopeptide and di/tripeptide transporter) required for valaciclovir absorption. After absorption, valaciclovir will be hydrolyzed to acyclovir. Cidofovir Some viruses do not have thymidine kinase, so resist the action of acyclovir. Cidofovir is already phosphorylated, so no need for the kinase, then this will be phosphorylated by cellular thymidine kinase to give the active Cidofovir triphosphate. Could be more toxic than acyclovir (why?). Mimic Other antiviral agents Are phosphorylated equally by viral and cellular thymidine kinase, so they are more toxic than acyclovir. The first nucleoside-based antiviral agents (inhibit both viral DNA polymerase and thymidylate synthetase). AIDS Acquired Immune Deficiency Syndrome caused by human immunodeficiency virus (HIV virus). Immune deficiency because the virus attacking the T- cells which are crucial to the immune system. Acquired because with weakened immune system, the patient will be more susceptible for opportunistic secondary diseases. infection by opportunistic pathogens (e.g. pneumonia, TB) ultimately kills the host not the virus itself in most of the cases. HIV virus Is one of the RNA retroviruses. Two types: HIV-1: is responsible for AIDS in America, Europe, and Asia. HIV-2: prevalent in Western Africa. Most clinically useful antiviral agents against AIDS are either: Reverse transcriptase inhibitors. HIV protease inhibitors. Life cycle of HIV virus HIV protease is required to process the transcribed and translated proteins for the new virions Inhibition of RT would prevent conversion of viral RNA genome into DNA for incorporation into the host’s replicatory system Antiviral agents against HIV Until 1987, no anti-HIV drug was available. Extensive studies carried out on the life cycle of HIV have led to identifying possible drug targets within the viral cell: Reverse transcriptase. Protease. Unfortunately, HIV undergoes mutation extremely easily, which results in rapid development of resistance. For that, current therapy depends on the use of combination of reverse transcriptase inhibitors and protease inhibitors. The ideal anti-HIV agent Must have high affinity for its target. Activity range in picomolar. Be effective in preventing the virus multiplying and spreading. Show low activity against host enzymes. Safe and well tolerated. Has a broad antiviral activity. It must be inexpensive since it will be used for the life time of the patient. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) This enzyme is unique to the virus so it is an ideal target. However, it still a DNA polymerase like, so there is a possibility that its inhibitor might affect the cellular DNA polymerase. Nucleoside-like drugs have been proved as useful antiviral agents: Nitrogen base + Deoxyribose sugar. Should be phosphorylated three times (by cellular kinases) to form the active nucleotide triphosphate. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) O O NH NH N N O HO HO O O H H OH H H Deoxythymidine H H Non-nucleophilic Azido group H H N3 H H Zidovidine (AZT) O GROWING CHAIN OF NEW STRAND GROWING CHAIN OF NEW STRAND GUANINE GUANINE O O O O O P REVERSE TRANSCRIPTASE O H O H OH H O O P O H O H H O O O H P H O NH H N O NH N O O O H H N3 H H O O O P O P O O O P O O O H H N3 H H RT uses zidovidine triphosphate in place of thymidine triphosphate as complementary base to Adenine In template strand NON-NUCLEOPHILIC FUNCTIONAL GROUP O 3' 5' - O O O O - P O O O- O P O O O O O P O O O O A G O C A T C G T O O O O O O P O O OH O N3 O O- O P No further nucleic acid extension O O - O P O O - P O O O- 5' 3' Other NRTIs Non-nucleoside reverse transcriptase inhibitors (NNRTIs). They are hydrophobic molecules bind to the allosteric binding site which is hydrophobic in nature (noncompetitive reversible inhibitors). Rapid resistance emerges due to mutation in the NNRTI binding site. First generation Second generation Allosteric site vs. catalytic site Nevirapine binding to the NNRTI allosteric site NNRTI binding transmits a conformational change through the RT protein Relative orientation of nucleophilic 3’-OH from growing strand and electrophilic 5’-phosphate from nucleotide triphosphate substrate altered and can no longer form a bond – elongation stopped Viral protease Has a broad substrate specificity, can cleave variety of peptide bonds in viral polypeptides. Mostly it can cleave the peptide bond next to proline residue and an aromatic residue (phenylalanine and tyrosine). This cleavage (next to proline) is not common with mammalian proteases such as renin and pepsin….. This results in better selectivity against HIV protease over the mammalian proteases. HIV-1 protease is 50% similar to HIV-2 homologue, the differences is far from the active site so high possibility to get inhibitors against both a the same time The role of the Proline residue Tertiary amide Induces turn in the backbone Mechanism of hydrolysis by protease enzyme Targeting HIV protease HIV protease is a much smaller enzyme than the equivalent host aspartate proteases. Cleaves substrates at the N-terminal to proline residues unlike mammalian proteases. Peptides from infected cells suggested that Tyr-Pro sites were the likely cleavage sites. Rationale for inhibitor design was based on PhePro or Tyr-Pro motif. HIV Protease Inhibitors (PIs) Are not prodrugs and do not need to be activated. So can be tested in-vitro to test activity (IC50….the concentration of drug required to inhibit the enzyme by 50%) especially after knowing that viral protease can easily isolated. Low IC50 does not mean a good antiviral activity (Why?). Most of them are derived from peptide lead compounds. HIV Protease Inhibitors (PIs) They are oligopeptides in general: Less well absorbed. Susceptible to first pass metabolism by cytochrome P- 450, which may results in drug-drug interactions with many other drugs taken by AIDS patients such as ketoconazole, rifampicin and astemizole. Rapidly excreted (Why?). High plasma protein binding (Why?). The Lead PI N and C-terminal protecting groups prevent hydrolysis by exopeptidases HO O O OH Asn HN NH O NH2 Pro O HN O O NH H N N O O O Tyr OH O Possible structural modification on the lead PI The size of the compound: di, tri, tetra, or pentapeptide. The amino acid motif: tyr-pro or phe-pro. The nature of C and N-terminal protecting group. The use of proline analogues Improve binding To the active site Better water Solubility and availabiliy