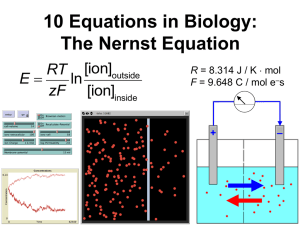
The Flu Network
Astrid.Ferlinz@lifetech.com
JCVI
Rockville
David
Wentworth
SMI
Stockholm
Steve
Glavas
Picture
Picture
ISS Rome
Gabriele
Vaccari
USDA –
NVSL
Ames, IA
Mary Lea
Killian
Picture
CDC
Atlanta
Catherine
Smith
SMI Smittskyddsinstitutet
ISS Istituto Superiore di Sanità
CDC Centers of Disease Control
JCVI J Craig Venter Institute
USDA – NVSL US Dep of
Agriculture – National Veterinary
Service Labs
Picture
Picture
Goals & Objectives:
Evaluate & define protocol
for Flu Typing
Share & discuss results
Make protocol/publication
publicly available
Rapid Influenza A virus typing on the
Ion PGMTM Sequencer
Influenza A Virus
RNA virus
Genome size: approx 13.5 kb
Comprised of 8 segments that encode up to 11 proteins
Transcriptase: cap binding
Transcriptase: elongation
Transcriptase: protease activity?
Haemagglutinin
Nucleoprotein: RNA binding – transport of vRNA
Neuraminidase: release of virus
Matrix protein 1: major component of virion
Matrix protein 2: Integral membrane protein – Ion Channel
Non-structural protein 1: RNA transport, translation, splicing
Non-structural protein 2: function not known
PathAmpTM FluA Pre-Amplification Reagents
Core consensus 5’
PCR primer
Core consensus 3’
RT/PCR primer
Influenza A virus genomic RNAs
•Highly specific Influenza A primer set (RT primer and PCR primer)
•High-fidelity master mixes for Reverse Transcription and PCR amplification of all 8
segments in a single tube
•Whole genome amplification
•Generates fragments that range in size from 900 bp to 2.4 kbp
Ion Torrent™ vs current workflow
Ion Torrent™
Current CE (Sanger)
Throughput
10 samples/run (Ion 314™
chip)
1 sample/run
Data
Whole genome
H and N genes only
TAT
18 hrs/10 samples
2-3 Days
Use
RUO, research, epidemiology,
monitoring
Screening (subset of samples only)
Specificity
Sub-types, mixed infections
Main H/N variants only; single
infections
Cost
115 Euro/sample*
>€200 (H/N genes only)
Ion PGM™ Sequencing allows:
Deeper understanding of genetic landscape and re-assortments within the
Influenza A genome
Workflow
60 min
3 hrs
RNA Extraction
Reverse Transcription
PCR
Optional: Agarose gel
analysis
MagMax™-96 Viral
RNA Isolation Kit
20 µl with up to 8 µl
Sample
50 µl with ALL RT
product
8 segments from 2400
to 900 bp size
30 min
~6 hrs
5 hrs
3 hrs
Amplicon clean up &
Quantitation
DNA library prep
Enrichment of library
Sequencing
MagMax™ Viral RNA
Isolation Kit
Nanodrop
Ion Xpress™ Plus
5
Fragment
Library Kit
Ion PGM™ 200
OneTouch™ Template Kit
Ion 314™/316™ Chip
Average Coverage
Results & Comparison with CE Sequencing
Ion 316™ Chip
Data provided by S Glavas, SMI,
Stockholm
Decrease of segment lengths
Clinical isolates (H1N1, 2009 H1 pandemic, H3) run in 10-plex on Ion 316™ Chip
simultaneously
Results were in 100% concordance with previous CE- data (H and N only) and
were also confirmed by CE-sequencing of all 8 segments
Application note
Released April 2013
Collaborator & internal R&D data
7
Data analysis plugin
Launched in IC in April 2013
by collaborators from SMI
Sequencing of the
H7N9 virus during
China Outbreak
Flu Season Winter/Spring 2013
PathAmpTM FluA PreAmplification Reagents
were used to sequence
whole-genome of H7N9
Highly accurate and
sensitive results from both
swab samples and
isolated virus samples
Detection of mixed
infections
Publication submitted
Thank you !
Start sequencing now at
lifetechnologies.com/iontorrent
For Research Use Only. Not for use in diagnostic procedures.
© 2013 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are
the property of Life Technologies Corporation and/or its affiliate(s) or their respective owners.
Limitations and Disclaimers
For Research Use Only. Not for use in diagnostic procedures.
Limitations and Disclaimer: Life Technologies Corporation takes no corporate position on the use of
selection methods in IVF and prenatal settings though we acknowledge that people disagree about its
appropriate use and it should ALWAYS be provided with full and informed, non-coerced prior informed
consent.
The PGM™ System and equipment used herein is RUO marked and may not be GMP. The results
shown may not represent actual performance in an IVF or any other setting. LTC does not assure or
endorse the use of its methods in ANY clinical setting outside of those that have been reviewed by the
FDA or similar oversight body.
© 2013 Life Technologies Corporation. All rights reserved.
The trademarks mentioned herein are the property of Life Technologies
Corporation and/or its affiliate(s) or their respective owners