Pre-implantation Genetic Testing
on Ion Torrent™ PGM™ System
Alain Rico
FALCON team
Global Clinical Research Program Manager
Picture: www.bio-itworld.com
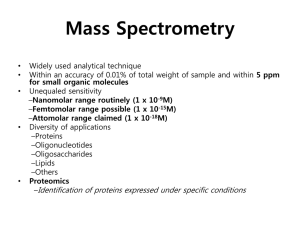
Described* Pre-implantation Genetic Testing
workflow on Ion Torrent™ PGM™ System
Alain.Rico@lifetech.com
< 24 hours workflow
– 15 hours (3 hours hands on)
– 3 hours total WGA
70€ per embryo
– 32 embryos per run
– The cost of reagents will reduce over time
Same run, more info
– Aneuploidy
– Mitochondrial genome
– Mutations
* user data. Wells et al., submitted
Described* Pre-implantation Genetic Testing
Workflow on Ion PGM™ system
Picture: www.bio-itworld.com
* user data. Wells et al., submitted
Select Cell(s)
Ion OneTouch™ 2
Whole Genome Amplification
used for
Fragment library (100 bp insert)
+
Target specific amplicon
Ion 316™ Chip
Up to 32 embryos
100 bp reads
Custom Script
or
Ion Reporter™
Day 1
1pm
O/N
Day 2
1pm
Single Cell (Low Pass)
Whole Genome CNV Analysis
Ion PGM™
150K reads
aCGH
Data courtesy Wells et al., submitted
Ion Reporter™ Software
Detect chromosomal
aberrations with
built-in informatics
baseline controls
1
Low-pass whole-genome
sequencing
2
Analyze with aneuploidy
workflow
3
Visualize aneuploidy through
customize IGV karyotype view
4
Create your interpretive report
Ion Reporter™ Software
Sample 1: -5, -22
Average of 0.01X coverage per sample – 100% concordance with aCGH
Data courtesy Dr Fiorentino, 10plex on Ion 316™ chip
Ion Reporter™ Software
Sample 2 : -13, +14, +15
Average of 0.01X coverage per sample – 100% concordance with aCGH
Data courtesy Dr Fiorentino, 10plex on Ion 316™ chip
Mitochondrial genome
IGV data courtesy Wells et al., submitted
Pre-implantation Genetic Diagnosis
Ex: CFTR carrier typing
IGV data courtesy Wells et al., submitted
www.lifetechnologies.com/aneuploidy
Listen to Prof. Wells
www.youtube.com/iontorrent
Interview on BBC Radio 4
http://www.guardian.co.uk/science/
2013/jul/07/ivf-baby-born-geneticscreening
Mapping the genes of a baby
before implantation
New Genetic Test May Boost IVF Results
First Child Born Following Embryo Screening With
New Genome Analysis Technique
Limitations and Disclaimers
For Research Use Only. Not for use in diagnostic procedures.
Limitations and Disclaimer: Life Technologies Corporation takes no corporate position on the use of
selection methods in IVF and prenatal settings though we acknowledge that people disagree about its
appropriate use and it should ALWAYS be provided with full and informed, non-coerced prior informed
consent.
The PGM™ System and equipment used herein is RUO marked and may not be GMP. The results
shown may not represent actual performance in an IVF or any other setting. LTC does not assure or
endorse the use of its methods in ANY clinical setting outside of those that have been reviewed by the
FDA or similar oversight body.
© 2013 Life Technologies Corporation. All rights reserved.
The trademarks mentioned herein are the property of Life Technologies
Corporation and/or its affiliate(s) or their respective owners