PROVING THE PRESENCE OF ISOTOPES
advertisement

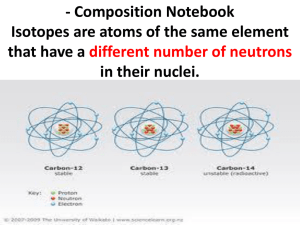
PROVING THE PRESENCE OF ISOTOPES By Rania, Cheryl, ZhenJie and Ivy Definition of Isotopes Isotopes are two or more of the Isotopes two or more atoms that different are species of the same have the same numbereach of protons chemical element havingbut different of neutrons. different number atomic mass (mass number). InIsotopes other words, havehave the same of an they element nuclei atomic numbers (Z) with the same number of protons (the But different numbers (A). same atomicmass number) but different numbers of neutrons. Examples of Isotopes Neutrons Protons Nucleus Carbon Similarities? Differences? Discover the Presence of Isotopes Joseph John Thomson was the one who discovered isotopes. Born in 1856 in Manchester, he studied at Trinity College. He was also the one who discovered the electrons. The discovery of the isotopes are also related to that of the electrons. Discover the Presence of Isotopes Wonder about the rays that moves towards the cathode? They are positively charged. Experiments showed that these rays: consist of massive particles and the charge of the positive particles is the same in magnitude as the electrons. Discover the Presence of Isotopes Through calculations done, it was clear that there are groups of atoms that are: of the same element but different mass which is not due to the number of positively or negatively charged particles. We shall use Aston’s better method (than Thomson’s) to explain what is being done. ASTON, Francis William ASTON, FRANCIS WILLIAM. (1877-1945) British chemical physicist: he invented the mass spectrograph, which could determine the existence of isotopes in an element. Proving the existence of isotopes Using Aston’s mass spectrograph. HOW? Mass spectrometry is also used to determine the isotopic composition of elements within a sample. A very sensitive instrument as: Differences in mass among isotopes are very small; and Less abundant isotopes are very rare. The Mass Spectrometer Electromagnetic force is used to separate different isotopes of the same element. The Mass Spectrometer Before letting the spectrometer determine the isotope mass ratio, the substance is being turned into gaseous form as well as becoming electrically charged (ions). The Mass Spectrometer After the substance is charged, it is being repelled into the spectrometer. The Mass Spectrometer The analyzer (which exerts electromagnetic force) bends the ray of ions, as there is a (positive) charge. The Mass Spectrometer As some isotopes are heavier, they will be repelled more from the magnetic force. Hence the resulting path of the heavier isotopes are less bent. The Mass Spectrometer As some isotopes are heavier, their speed will become slower than those which are lighter due to the repelling force. The Mass Spectrometer The streams of sorted ions are passed from the analyzer to the detector, which records the abundance of each ion type. The Mass Spectrometer The result will be calculated with the massto-charge ratio, as the charge is known. The Mass Spectrometer This information is then used to determine the chemical element composition of the original sample, and the isotopic composition of its components. The Mass Spectrometer Taking sodium chloride as an example… What Aston Showed Us Over 50 elements consisted of atoms of: the same atomic number but different relative atomic mass however the differences are similar in pattern. The apparent deviations of relative atomic masses of the elements from integer results imply the presence of isotopes. After that… Shortly after Aston’s discovery, W.D. Hawkins and his students from The University of Chicago used fractional distillation to separate Mercury vapour into six isotopes. This experiment led to a series of more discoveries about isotopes in the following year, as they followed in Aston’s footsteps. References http://www.books.google.com.sg/books?id=wKzJTBZh20 wC&pg=PA234&dq=evidence+of+the+presence+of+iso topes&sig=ACfU3U0UXYEf1FVrwi10AkbSfWbg_QB0OQ# PPA234,M1 - Last accessed, 9th July 2008 The Cambridge Dictionary of Scientists - Last accessed, 5th July 2008 http://en.wikipedia.org/wiki - Last accessed, 9th July 2008 http://www-outreach.phy.cam.ac.uk/camphy /positiverays/positiverays_index.htm - Last accessed, 17th July 2008