Polymers Amiders - Clydebank High School
advertisement
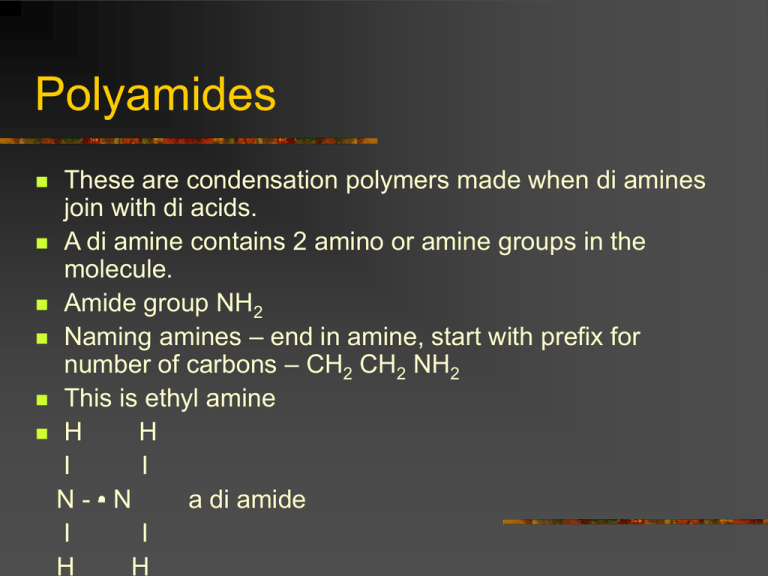
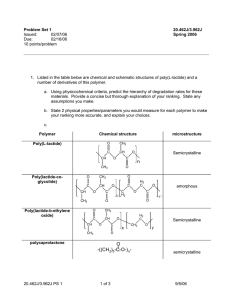
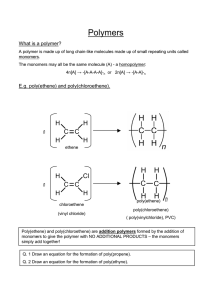
Polyamides These are condensation polymers made when di amines join with di acids. A di amine contains 2 amino or amine groups in the molecule. Amide group NH2 Naming amines – end in amine, start with prefix for number of carbons – CH2 CH2 NH2 This is ethyl amine H H I I N-• -N a di amide I I H H Polyamides Example H H I I N-• -N + I I H H O O II II C–Δ-C I I HO OH H H O O I I II II N -•- N – C – Δ – C + H2O I I H OH H O I II The N – C is the amide link. More info! Nylon is a polyamide. It is a thermoplastic. We can make different polyamides by altering the number of carbons in the chain. They can be used for ropes, clothing, pulleys. The N-H and C=O groups in molecule allow for H bonding. Resulting in very strong polymer. Synthesis Gas This is a mixture of CO and H2. It is made by steam reforming methane. We use synthesis gas to make methanol. This is oxidised to form methanal. Methanal is used to make thermosetting plastics. The methanal is used to cross link the polymer chains in a condensation reaction. This gives a rigid 3D structure. Examples – bakelite, urea – formaldehyde resin. Thermosetting plastics. New Polymers Kevlar It is an aromatic polyamide which is extremely strong because the linear molecules are rigidly packed. It is formed by condensation polymerisation. Hydrogen bonds form between polymer sheets. It is very strong and is used to replace steel in car tyre radials, bullet proof vests, fencing jackets, motor bike clothing. It is abrasive resistant. Poly( ethenol) This is a soluble plastic. It is made by ester exchange: Ethenyl ethanoate –> addition polymerisation –> poly( ethenyl ethanoate) –> ester exchange –> poly( ethanol). It is used for laundry bags, dissolving stitches, protective coatings over new cars. The % of acid groups that are removed in the reaction affects the intermolecular forces and therefore the solubility. Poly ( ethyne) This is an addition polymer made from ethyne. It can be treated to enable it to conduct electricity. This is due to the delocalised electrons along the chain. Conducting polymers make the membranes for loud speakers. Poly ( vinyl carbazole) This is an addition polymer. It is exhibits photoconductivity – it conducts much better when light shines on it. It is used in photocopiers. Biopol This is a natural polymer that is biodegradable. It is made by certain bacteria. It can be broken down by bacteria in the soil. Low Density Poly (Ethene) LDPE This can be altered to be photo degradable. It breaks up in sunlight. Carboxyl groups are added to the chain that can absorb UV light. The trapped energy causes bonds, around the COOH group, to break and the polymer breaks into smaller pieces.