Enzyme Notes
advertisement

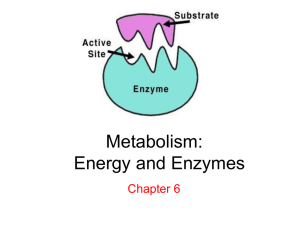
Chemical Reactions Chemical Reactions and Enzymes chemical reaction: process that changes one set of compounds (reactants) into another set of compounds (products) A. example: wood + oxygen carbon dioxide + water + energy Reactants Products Reactants: • The elements or compounds present at the beginning of a chemical reaction Products: • The elements or compounds produced at the end of a chemical reaction Chemical reactions: • Always involve changes in the chemical bonds that joins atoms in compounds. How many molecules are in a chemical formula/equation? Photosynthesis: Put a box around the products and circle reactants carbon dioxide + water glucose + oxygen Reactants Products chemical equation: CO2 + H2O C6H12O6 + O2 Reactants Products 6CO2 • This Molecule is called…. • Carbon Dioxide • Number of molecules…. •6 • Number of Carbon atoms…. •6 • Number of Oxygen atoms… • 12 6H2O a) This Molecule is called…. • Water • Number of molecules…. •6 • Number of Hydrogen atoms…. • 12 • Number of Oxygen atoms… •6 Glucose: C6H12O6 b) Glucose or Sugar: • Number of molecules…. •1 • Number of Carbon Atoms… •6 • Number of Hydrogen atoms…. • 12 • Number of Oxygen atoms… •6 6O2 c) Oxygen…. • Number of molecules…. •6 • Number of Oxygen atoms… • 12 • How many TOTAL ATOMS of each element are present on the REACTANTS side of the reaction: • Carbon: ___________ 6 • Oxygen: _____________ 18 • Hydrogen: ____________ 12 • How many TOTAL ATOMS of each element are present on the PRODUCTS side of the reaction: • Carbon: ___________ 6 • Oxygen: _____________ 18 • Hydrogen: ____________ 12 • If you did your math correctly in step d. and e. you have observed a fundamental scientific principle called THE CONSERVATION OF MATTER. • Using the information you gained in part d. and e. explain what the conservation of matter is using the sentence starter provided: • The principle of the conservation of matter is demonstrated by the chemical reaction above because: C. Conservation of matter: During a chemical reaction, atoms are not created or destroyed – just rearranged. Therefore, chemical equations must be balanced so there is the same number of atoms on both sides of the equation. balancedequation: chemical equation chemical CO22 ++ H C H O + O 6CO 6H O C H O + 6O 2O 6 12 6 2 2 2 6 12 6 Apply what you’ve LEARNED a.) 2 H2O2 2H2O + O2 • # of reactant molecules: 2 • # of product molecules: 3 • What are the molecules: • Hydrogen Peroxide • Water • Oxygen Apply what you’ve LEARNED b.) 2 H2O2 2H2O + O2 • # of reactant atoms: 8 • (4 H and 4 O) • # of product atoms: 8 • (4H, 2O, 2O) Apply what you’ve LEARNED c.) 2 H2O2 2H2O + O2 • # of reactant elements: 2 • # of product elements: 2 • What are the elements: • Hydrogen • Oxygen II. Energy in Reactions: • Energy is absorbed or released whenever chemical bonds form or are broken. Chemical reactions that Release energy…. • Often Occur Spontaneously (without warning) • Example: Explosion gun powder or fireworks Chemical reactions that Absorb energy…. • Will not occur without a source of energy. • Example: Instant Ice Packs or Photosynthesis. • The speed of a reaction depends on whether is absorbs or releases energy. Chemical Reactions and Enzymes III. Label the graph: Activation energy Products Activation Energy Reactants Energy Absorbing Reactants Products Energy Releasing A. All reactions require some energy to start: activation energy. Example: if some reactions that release energy did not require activation energy, what could happen to the pages of your text book as you sit here reading? They could spontaneously combust into flames. B. catalyst: substance that speeds up a chemical reaction by lowering the activation energy Catalysts found in living things are called enzymes C. Enzymes •provide a site where the reactants of a chemical reaction can be brought together •are not used up or changed during the chemical reaction •active site: part of the enzyme where the reactants bind (stick) •reactants: molecules at beginning of a chemical reaction. Called the substrate when they encounter an enzyme. •substrate only fits into the active site of the correct enzyme (like a key and lock) Labeled diagram: Enzyme Active Site Reactants III. How does an enzyme work? A. substrate binds to the enzyme Enzyme Substrate Active Site Reactants III. How does an enzyme work? A. substrate binds to the enzyme Enzyme Substrate Active Site III. How does an enzyme work? B. reactants converted to product Enzyme Product Active Site III. How does an enzyme work? C. products are released – enzyme is free to bind new substrate Enzyme Product Active Site IV. Enzymes only work in specific conditions A. temperature: heat=destroy enzyme, cold= slow enzyme down B. pH: changes the shape of the enzyme and its active site C. coenzymes: make enzymes work better D. inhibitor molecules: block substrate from entering active site pH Change: Enzyme Product Active Site No Product Made Inhibitor Molecules: Enzyme Product Active Site Enzyme: Peroxidase in liver cells Reactants: Hydrogen peroxide Enzyme Reactants Hydrogen Peroxide Active Site Enzyme: Peroxidase in liver cells Reactants: Hydrogen peroxide Enzyme Reactants=Substrate Active Site Enzyme: Peroxidase in liver cells Products: Water and Oxygen Enzyme Product O2 H2O Active Site Enzyme: Peroxidase in liver cells Products: Water and Oxygen Enzyme More Hydrogen Peroxide Active Site O2 H2O Product Macromolecules • Carbohydrates • Lipids • Proteins • Nucleic Acids Carbohydrates • Made of Sugar • Main Source of energy • Examples: Sugar Lipids • 1 Glycerol & 3 Fatty Acids • Stores energy • Examples: Fats, Cholesterol Protein • Amino Acids • Makes up structures in body • Examples: Hair, muscles, skin, bones, etc. Nucleic Acids • Nucleotides • Store genetic material • Examples: DNA, RNA Chemical Reactions and Enzymes D. Practice: balance the following equations 2) _2 Cu + _1 S 1_ Cu2S 1) 4_ Na + _1 O2 _2 Na2O 3) _1 CuO + _1 H2 1_ Cu + _1 H2O