Make me a molecule
advertisement
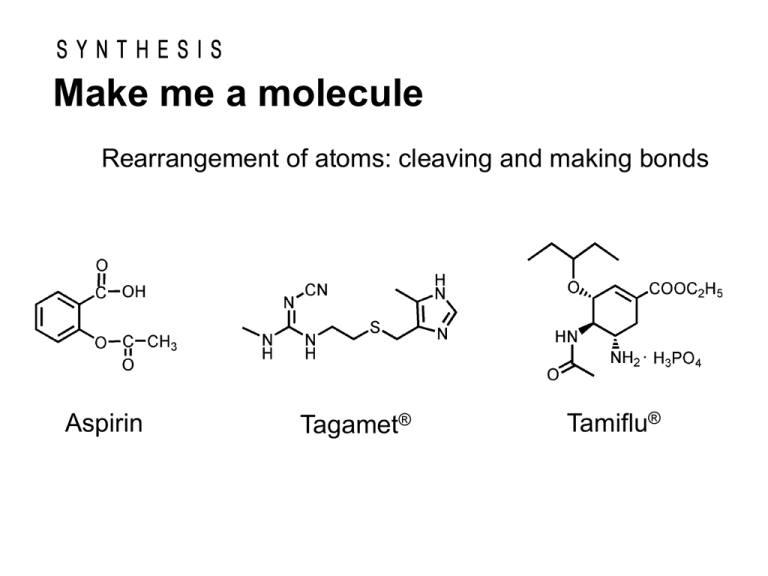
Make me a molecule Rearrangement of atoms: cleaving and making bonds Aspirin Tagamet® Tamiflu® SYNTHESIS Make me a molecule “Chemists make molecules… they study the properties of these molecules; …” “At heart of their science is the molecule that is made.” ― Roald Hoffmann― Constructing complex molecules is both an art and a science which stretches the chemist’s knowledge and insight, practical skills and imagination. Synthesis is the bedrock on which the chemical and pharmaceutical industries are built. In the beginning synthesis was unnecessary. Chemistry was founded upon the study of those materials which were available from natural sources. Many of the materials we use today are made by chemical synthesis. Most of the new compounds made today are organic. Organic Chemistry Organic Compounds Most of the new compounds made today are organic, that is, they are composed largely of carbon. C C H C C O C C C H N C O O C Cl C C C stable and versatile C N N C unstable Product e.g. paint and pigment vitamin synthetic fiber pesticide plastic explosive pharmaceutical Synthesis involves breaking and making new chemical bonds to create new chemical structures. e.g. Aspirin In 400 B.C., the Greek physician Hippocrates recommended chewing bark of the willlow tree to alleviate the pain of childbirth and to treat eye infections. *It is known in ancient Egypt . CH2OH Salicin active component Hydrolysis of salicin in aqueous acid gives salicyl alcohol, which can be oxidized to salicylic acid. Salicylic acid proved to be an even more effective pain reliever. Unfortunately, it causes severe irritation of stomach. O glucose CH2OH O H O C OH Saliciylic acid O H O In 1883, chemists at Bayer division of I. G. Farben in Germany prepared aspirin. It proved to be less irritating to the stomach than salicylic acid and also more effective in relieving the pain and inflammation of rheumatoid arthritis. Salicyl alcohlol O H3C C O C CH3 O C OH O C CH3 O Acetylsalicylic acid (Asprin) Organic Synthesis: 1. Carbon-Carbon Bond Formation 2. Functional Group Interconversion Efficiency and selectivity are important characteristics that have to be taken into account. [Efficiency: yields, number of steps] [Selectivity: chemoselectivity, regioselectivity, stereoselectivity] Organic Synthesis: Stereoselectivity 1. Carbon-Carbon Bond Formation 2. Functional Group Interconversion Organic Synthesis: Enantioselectivity 1. Carbon-Carbon Bond Formation 2. Functional Group Interconversion 분자식 입체이성질체(Stereoisomers) Cl C2H2Cl2 Cl C H C7H14 C C H H cis-1,2-dichloroethylene (bp: 60 oC) Cl H H Cl trans-1,2-dichloroethylene (bp: 48 oC) Me H H Me Me H Me cis trans CH2CH3 CH3CH2 C HI C 4H 9I CH3 (+)-2-iodobutane (bp: 119 oC) Chiral compounds “Enantiomer” C H C I CH3 (-)-2-iodobutane (bp: 119 oC) 아카티넬라 데코라 (하와이) 암피드로무스 (동남아시아) Chiral Compounds CH2CH3 CH3CH2 H C I C HI CH3 CH3 (+)-2-iodobutane (bp: 119 oC) (-)-2-iodobutane (bp: 119 oC) [ ]D = +15.9 [ ]D = -15.9 Enatiomers Optical isomers They are optically active. Some Physical Properties of the Stereoisomers of Tartaric Acid HO H NH2 HO (S) 도파 (R) N H2N H OH 심각한 부작용 파킨슨병 치료 H3C OH HOOC COOH H H N CH3 (S) 케타민 (R) Cl O O Cl 환각제 마취제 H3C CH3 H3C COOH (S) 페니실아민 (R) H3C CH3 HOOC H2N H H OH 결핵치료 HO OH N H NH2 돌연변이유도 관절염치료 H N CH3 (S,S) 에탐부톨 (R,R) N H H N HO 시력상실 O O H H O N N H O O (S) 탈리도미드 (R) O N N O H 기형아 생성 HO O O 진정제 H O H N H NH2H O (S,S) 아스파탐 (R,R) O ¾´¸À O H2N O OH (S) 아스파라진 (R) N H H2 NH2 HO H N O H2 ¾´¸À ´Ü¸À CH3 CH3 (S) 리모넨 (R) ·¹ ¸ó Çâ O N H H2N H O O ´Ü¸À O O ¿À·» ÁöÇâ OH Louis Pasteur was born on December 27, 1822 in Dole, in the region of Jura, France. Louis Pasteur Chemist Resolution of enantiomers in 1848 1822-1895 Twenty years later, J. H. van’t Hoff and J.-A. Le Bel independently explained the origins of enantiomers based on the tetrahedral nature of carbon bonding. “Chance favors only prepared mind.” -Louis Pasteur Resolution diastereomeric salts seperation by selective crystallization or some other means Once separated, acidification of the two diastereomeric salts with strong acid gives pure enantiomers and recover the chiral amine.



















![AL Chem Written Practical (Organic Chemistry) [F.7]](http://s2.studylib.net/store/data/005797652_1-4911d95dd6c8a0840f727bd387aa6027-300x300.png)