limestone
advertisement
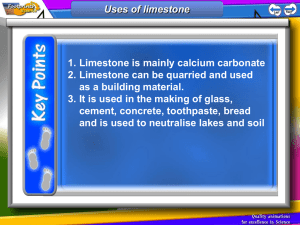
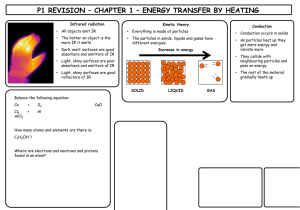
Limestone Limestone is a type of sedimentary rock that is mainly made up of a substance called calcium carbonate. Calcium carbonate has the chemical formula, CaCO3 Because it has many uses and can be used to make other products, limestone is a valuable resource. Basic properties of limestone The appearance of limestone can vary tremendously. Limestone is calcium carbonate and like any carbonate, it reacts with acids producing carbon dioxide gas. Carbon dioxide turns limewater milky. bubbles of CO2 Because it reacts with acids, it is sometimes added to lakes to remove acidity caused by acid rain. Limestone, chalk and marble are all forms of calcium carbonate CaCO3. Limestone Chalk Marble Limestone is a sedimentary rock and most of it (especially chalk) was formed from the remains of tiny sea creatures. This process took millions of years. Limestone and chalk often contain fossils. Where is limestone found in the British Isles? limestone areas There are also limestone cliffs on the coast in the Vale of Glamorgan Sedimentary rocks at Llantwit Major in the Vale of Glamorgan Layers of limestone and shale fossils from rocks on left fossils in the fallen limestone blocks prepared specimen – a Jurassic oyster (180 million years old!) More fossils found in limestone. A Jurassic ammonite from North Yorkshire. 180 million years old. A Silurian gastropod (snail) from Shropshire. 400 million years old. Important uses of limestone 1. As a building material Limestone has been used for thousands of years for buildings and roads. However, since it is attacked by acids it is easily weathered and eroded. 2. For making glass Glass is made by heating a mixture of limestone , sand and sodium carbonate (soda). 3. For making cement Cement is made by heating together powdered limestone and clay in a rotary kiln. Limestone and clay Rotating kiln Gas burners Cement Crusher Cement Mix with sand and water and chippings concrete for buildings etc. Mix with sand and water mortar for joining bricks together Summary – the uses of limestone Buildings and roads Mortar (cement + water + sand) Cement (heat with clay) Glass (heat with sand and sodium carbonate) CaCO3 Neutralise acidic soils and lakes Concrete (cement + sand + gravel + water) Limestone Quarrying The quarrying of limestone can have major effects on the environment. Limestone quarrying provides the raw materials for making many important substances such as glass and cement etc. It also provides employment for people and benefits the local economy. But what exactly are the disadvantages? Limestone Quarrying – some disadvantages An ‘eyesore’ in areas of the countryside Noise from blasting Loss of habitat for wildlife Dust etc. from lorries Heating limestone When limestone is strongly heated is begins to glow (limelight!) The thermal decomposition of limestone It decomposes to form calcium oxide (quicklime) and carbon dioxide gas. We can write a word equation: calcium carbonate calcium oxide + carbon dioxide or a symbol equation: CaCO3 CaO + CO2 This type of reaction, where a substance is broken down by heat is called a THERMAL DECOMPOSITION Calcium oxide (quicklime) is produced industrially using a lime kiln Kiln rotates to ensure that the limestone is mixed with the hot air Air + carbon dioxide Hot air in Limestone in Lime out heat calcium carbonate calcium oxide + carbon dioxide A thermal decomposition reaction a lime kiln Adding water to quicklime ‘slaking’ lime When water is added to quicklime (calcium oxide), the reaction produces much heat and slaked lime (calcium hydroxide) is formed. A reaction that produces heat is called an exothermic reaction. We can write a word equation: calcium oxide + water calcium hydroxide or in symbols CaO + H 2O Ca(OH)2 Calcium hydroxide solution is limewater which turns ‘milky’ with carbon dioxide. Slaked lime (calcium hydroxide) also has uses: to neutralise acidic soil to make lime mortar (often used to restore old buildings Heating other carbonates A number of other carbonates are decomposed in the same way as calcium carbonate when heated e.g. CuCO3 heat copper carbonate CuO + CO2 copper oxide + carbon dioxide (green) (black) heat zinc carbonate zinc oxide + carbon dioxide (Zinc oxide is yellow when hot, white when cool) ZnCO3 Both these reactions are again examples of a thermal decomposition. ZnO + CO2