C1_Chemistry_Summary_Topic_2
advertisement
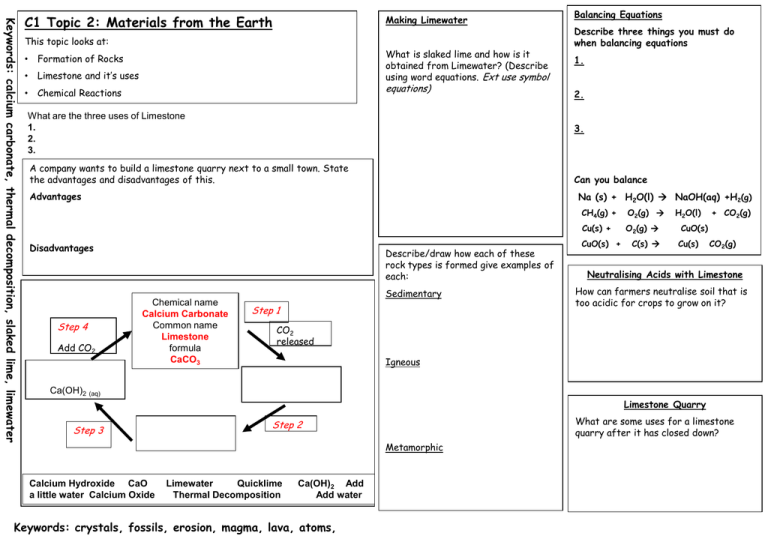
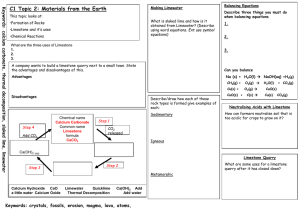
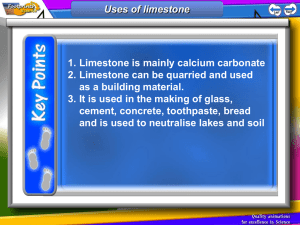
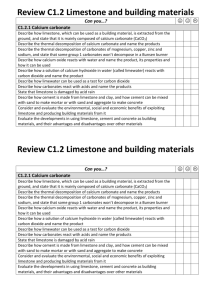
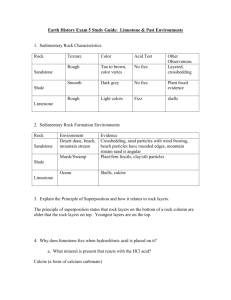
Keywords: calcium carbonate, thermal decomposition, slaked lime, limewater C1 Topic 2: Materials from the Earth Making Limewater This topic looks at: • Formation of Rocks • Limestone and it’s uses • Chemical Reactions What is slaked lime and how is it obtained from Limewater? (Describe using word equations. Ext use symbol equations) What are the three uses of Limestone 1. 2. 3. 1. 2. Can you balance Advantages Na (s) + H2O(l) NaOH(aq) +H2(g) Disadvantages Add CO2 Describe three things you must do when balancing equations 3. A company wants to build a limestone quarry next to a small town. State the advantages and disadvantages of this. Step 4 Balancing Equations Describe/draw how each of these rock types is formed give examples of each: Chemical name Calcium Carbonate Common name Limestone formula CaCO3 Sedimentary Step 1 CH4(g) + O2(g) Cu(s) + O2(g) CuO(s) + C(s) H2O(l) + CO2(g) CuO(s) Cu(s) CO2(g) Neutralising Acids with Limestone How can farmers neutralise soil that is too acidic for crops to grow on it? CO2 released Igneous Ca(OH)2 (aq) Limestone Quarry Step 3 What are some uses for a limestone quarry after it has closed down? Step 2 Metamorphic Calcium Hydroxide CaO a little water Calcium Oxide Limewater Quicklime Thermal Decomposition Ca(OH)2 Add Add water Keywords: crystals, fossils, erosion, magma, lava, atoms,