Acid and Base Jeopardy
advertisement

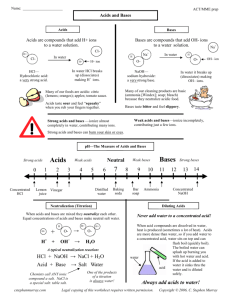
Choose a category. You will be given the answer. You must give the correct question. Click to begin. Click here for Final Jeopardy Acid/Base Indicators Properties Acid/Base of acids/bases reaction pH scale 10 Point 10 Point 10 Point 10 Point 10 Point 20 Points 20 Points 20 Points 20 Points 20 Points 30 Points 30 Points 30 Points 30 Points 30 Points 40 Points 40 Points 40 Points 40 Points 40 Points 50 Points 50 Points 50 Points 50 Points 50 Points Other Substance turns litmus paper red Acid Substance that turns litmus paper Blue Bases Substance that turns phenolphthalein bright pink Bases Phenolphthalein is clear or coudy in this solution Acids Substance that turns blue litmus paper blue and red litmus paper red A neutral substance. Water pH scale 0 1 C 2 3 4 5 6 D 7 B 8 9 E 10 11 12 13 14 A a) What letter would represent a weak acid? b) What letter would represent a strong acid? D Weak acids have a pH around 3-6.9 C Strong acids have a pH around 0-3 pH scale 0 C 1 2 3 4 5 D 6 7 B 8 9 10 E What letter would represent a strong base? 11 12 13 A 14 A Strong bases have a pH around 10-14 pH scale 0 1 C 2 3 4 5 6 D 7 B 8 9 10 11 12 E a) What letter would stomach acid represent on the pH scale? b) What letter would soap represent on the pH scale? 13 A 14 C Stomach acid contains HCl which is a strong acids E Soap is a weak base pH scale 0 1 C 2 3 4 5 6 D 7 B 8 9 E 10 11 12 13 14 A If we combine substance C and A, what letter would correspond the to correct pH. B Combining a strong acid and a stong base will neutralize each other and create a neutral substance. pH scale 0 1 C 2 3 4 5 6 D 7 B 8 9 E 10 11 12 13 14 A If we combine substance C and E, what letter would correspond the to correct pH. D Combining a strong acid and weak base will create a weak acid. Substances that tastes sour Acids (lemons, limes, oranges) Substance that is slippery Bases (soap is a weak base) Substance that tastes Bitter Base Substance that emulsifies fats and oils. Base For example, soap breaks apart fats and oils to make you’re your dishes clean. Substance that dissolves in water to produces positive hydrogen (H+) ions Acids break apart in water to produce H+ ions. Substance that dissolves in water to produces hydroxide (OH-) ions. Bases produce OH- ions. OH- combines with a H+ from acids to form water When you combine an acid and a base, what do you form? 2 neutral substances. Water and a salt What type of reaction is a neutralization reaction? Double replacement reaction. Finish the reaction. 2HBr + Ba(OH)2 ____2 + 2___ Neutralization reaction 2HBr + Ba(OH)2 BaBr2 + 2H2O a type of salt water a) What is heartburn? b) What can you do to relieve the discomfort? a) Stomach acids that enter the esophagus and eat away at the tissue which causes discomfort. b) An antacid will neutralize the stomach acid and relieve the discomfort. a) Acids always start with what? b) Bases usually end with what? a) Acids start with H. For example: HCl and H2SO4 Hydrochloric and sulfuric acid b) Bases usually end with OH For example NaOH and KOH What causes acid rain? Cars burn gasoline to produce NO2 Factories and power plants burn coal to produce SO2 Write down the Jeopardy question and answer. Hand in before the test for extra credit. What are 2 things you can do to prevent acid rain? Walk more and ride your bike instead of having your parents give you a ride. Carpool or take a bus. (etc) Use less energy. Turn off the lights when leaving a room or turn off your computer at night. (etc) What are 2 ways companies are reducing the amount of acid rain? Smoke stack scrubbers: Spray CaO and water on the smoke, which reacts with the SO2 to prevent it from entering the atmosphere. Catalytic converters are in the exhaust system in cars. They turn harmful chemicals into less harmful ones. If there is a lake that is acidic, what can we do to make it livable for aquatic organisms. Why? Spraying a base such as calcium carbonate (chalk) will neutralize the acid, creating salt and water, to make it livable for aquatic organisms. Make your wager What is the difference between a strong acid and a weak acid? This is not the EC question Strong acids break apart completely when put into water while weak acid only partially break apart. Good luck on the Chemistry test!