Alkynes Group
advertisement

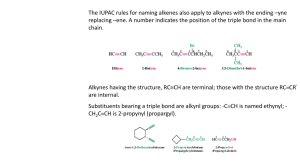
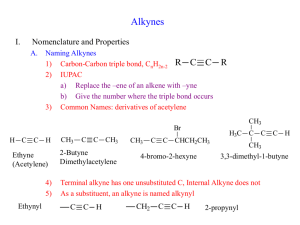
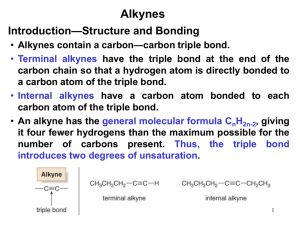
Alkynes Group Alkynes is hydrocarbons which respectively have carbon-carbon triple bond functional groups. NAMING ALKYNES Alkynes end in the suffix -yne, and are numbered in the same manner as alkenes, with the first carbon of the alkyne being numbered to specify its position. The triple bond is given the lowest number possible. Compounds containing double and triple bonds are named with the suffix -enyne. In this case the numbering begins at the side of the chain that is closer the multiple bond. If there is a choice the double bond gets priority. Of course there is no cis or trans Z or E in alkynes. Example: Penta-1,3-diyne Hexa-1,5-dien-3-yne Hex-1-ene-3,5-diyne You must relate where each multiple bond is on the carbon chain. Simply insert the position number in front of the appropriate suffix. Example 3-penten-1-yne Longest chain? 5, therefore pent Carbon alkyne starts on? In this case, the end carbon of the triple bond gets a 1 because it is closer to the end of the longest chain than the double bond Carbon alkene starts on? 3 Nomenclature • IUPAC: use the infix -yne- to show the presence of a carbon-carbon triple bond. 4 3 2 1 2 3 4 6 7 5 1 2 3 4 6 7 5 1 3-Methyl-1-bu tyn e 6,6-D imethyl-3-hep tyn e 1,6-Hep tadiyne • Common names: prefix the substituents on the triple bond to the word “acetylene”. IUPAC n ame : 2-Bu tyn e 1-Bu te n -3-yn e C omm on n ame : D i me th ylace tyl en e Vi nyl ace tyl e n e SOME EXAMPLES: 1-octen-5-yne The double bond is nearest to the end of the longest chain, so it receives numbering priority. 5-ethyl-3-heptyne Just like alkenes, the position of the triple bond goes before the name of the parent chain Try to give name of the compounds bellow: _________________________ _________________________ _________________________ _________________________ _________________________ Properties of Alkynes * Physical state The first three members (ethyne, propyne and butyne) are colourless and odorless gases. Due to the presence of phosphine as an impurity ethyne (acetylene) has garlic smell. The next eight members are liquids, and higher members are solids under normal conditions of temperature and pressure. * Solubility Alkynes are insoluble in water, but are fairly soluble in organic solvents such as, alcohol, ether, acetone etc. * Melting Points The melting and boiling points of alkynes increase with molecular mass. Uses of Alkynes Alkynes are generally used as the starting materials for the manufacture of a large number of organic compounds of industrial importance such as, chloroprene, vinyl chloride etc. Isomerism in Alkynes Positional Isomerism It is due to the different arrangement of carbon atoms in the chain i.e., straight chain or a branched-chain e.g., It is due to the difference in the location of the triple bond, i.e., Polymers Polymers: A large molecule formed by the repetitive bonding together of many smaller molecules called monomers. Many simple alkenes undergo polymerization reaction when treated with the proper catalyst. a). Addition Polymerisation This kind of polymerisation is typified by the presence of a carbon – carbon double bond in the monomer. Example: Polypropylene In addition polymerisation: • The polymer is the only product • Involves the opening out of a double bond • The conditions of the reaction can alter the properties of the polymer Another example: b. Condensation polymers A condensation polymer generally involves 2 monomers that have different functional groups. They also involve the elimination of water or another small molecule. Hence the term condensation polymer. Monomer A + Monomer B Polymer + small molecule (normally water). Common condensation polymers include polyesters (the ester linkage) and polyamides (the amide linkage as in proteins). Example: The example here is terylene, a polymer of benzene-1,4dicarboxylic acid and ethane-1,2-diol. The ester linkage is based around the reaction between an acid and an alcohol to form an ester + water. EXERCISES Predicting the repeating unit. Predicting the monomer from the polymer