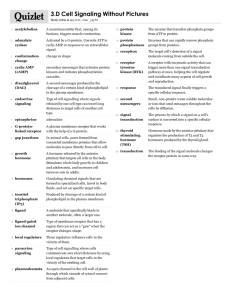
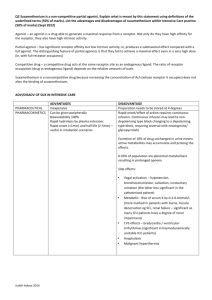
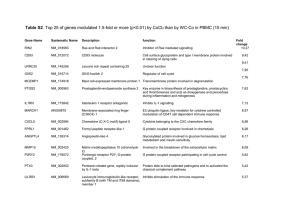
Physicochemical Properties of Drugs in relation to Drug Action

Physicochemical Properties of
Drugs in relation to Drug Action
Roselyn Aperocho Naranjo, RPh, MPH
USPF, College of Pharmacy rose_may26@yahoo.com
Understanding the
Modern Drug Design
Modern chemical techniques
Recent knowledge on disease mechanisms & receptor properties
Transportation of drugs in the body
Distribution of drug through different compartments
Metabolism of drugs in the liver and other organs
Structural characteristics of the receptor
Acid-base chemistry
Availability of the computer software that determines the three-dimensional shape of the receptor in order to design new molecules that will give optimum fit to the receptor
History: General
Plant Source
Specific Plants are selected
Treatment for medical conditions
Crude extracts
History: General
Plant Source
Structure of
Natural products
Specific Plants are
Treatment for medical conditions selected
Selective changes in the molecule
Crude extracts
Selected molecules are changed due to:
Reduction of undesirable pharmacologic response of the drug
(side-effect)
Obtain better drug response
Alter drug’s metabolism
Produce more cheaper and competitive supply of the product
Selected molecules are changed due to:
Example:
Morphine
Addiction effect reduced
Analgesic effect enhanced
Cocaine
Local Anesthetic
Effect enhanced
CNS effects reduced
Medicinal Chemistry in 1900’s
Phenothiazines – first synthesized as ANTIHISTAMINES
- Careful investigation led to discover its
TRANQUILIZING PROPERTY for the mentally ill patient.
Benzodiazepines – originated from an expected ring enlargement which resulted it to become a CNS RELAXANT
Overview
drug receptor Drug-receptor complex
Pharmacologic response
Overview
Drug Distribution
Oral Administration
Drug Distribution
Oral Administration
Solubility depends on:
Chemical Structure
Size of particles
Surface area
Nature of crystal form
Type of tablet coating
Type of tablet matrix
Drug Distribution
Oral Administration
Example:
CHLORAMPHENICOL
PRODRUG- inactive form but are easily metabolized in the liver to become active
CHLORAMPHENICOL PALMITATE
Protein Binding
Protein Binding
Can affect the drug’s effective solubility, biodistribution, half-life in the body and interaction with other drugs.
Control access to certain body compartments
Prolong the drug’s duration of action
Limit the amount of drug available for biotransformation and interaction with specific receptor sites.
Tissue Depot
Fats in the body which can be a storage for drugs
The more Lipophilic the drug is, the more it will stay in the tissue
Example:
Thiopental
Drug Metabolism & Excretion
Statistical Prediction of
Pharmacological Activity
Mathematical Model will explain many chemical processes
Three goals in Drug Design
• Predict biological activity in untested compounds
• define the structural requirements required to fit to a specific receptor
• design a test set of compounds
QSAR
Proposed by Crum-Brown and Fraser in 1865 to 1870
Certain modification in the molecular structure of a poisonous compound produces an important differences in their action
QSAR
Topological Descriptors
Alternate method in describing molecular structure which is based on graph theory using the bonds that connects between atoms
Combinatorial Chemistry
…to be continued
Prepare ¼ sheet of paper for the quiz



![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)