Trace Metal Biogeochemistry (Marine Bioinorganic Chemistry
advertisement

Trace Metal Biogeochemistry
(Marine Bioinorganic Chemistry) 12.755
Lecture 2
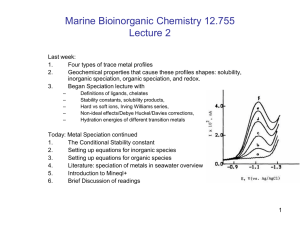
Last week:
1.
Four types of trace metal profiles
2.
Geochemical properties that cause these profiles shapes: solubility,
inorganic speciation, organic speciation, and redox.
3.
Began Speciation lecture with
–
–
–
–
–
Definitions of ligands, chelates
Stability constants, solubility products,
Hard vs soft ions, Irving Williams series,
Non-ideal effects/Debye Huckel/Davies corrections,
Hydration energies of different transition metals
Today: Metal Speciation continued
1.
The Conditional Stability constant
2.
Setting up equations for inorganic species
3.
Setting up equations for organic species
4.
Literature: speciation of metals in seawater overview
5.
Introduction to Minqel+ (if time)
6.
Brief Discussion of readings
1
2
3
Why are we talking about complexation chemistry?
• How do metals influence the biota (and carbon cycling) of seawater?
• To answer the question we have to understand:
- Natural organic-metal complexes:
FeL, CoL, NiL, CuL, ZnL, CdL
• What are the geochemical roles of these ligands?
1. Controls on “bioavailability”
- high affinity uptake systems
- ecological warfare between species
2. Protection from scavenging processes
3. Increases in solubility
• How do you study something at picomolar quantities which we don’t
know much about?
4
Chelators are
everywhere
5
FROM LAST WEEK:
Background Aquatic Chemistry of Trace Elements:
A marine water column context
Solubility Products: Example for Fe(OH)3(s)
Ksp= [Fe][OH]3 = 1042.7
Stability constants for metal complexes (where L is ligand, M is Metal):
K = [ML]/[M][L]
Ligands can include inorganic chemical species:
In oxic systems: OH-, CO32-,SO42-, Cl-, PO43-,
In anoxic systems add: HS-,, S2Ligands can also include organic chemical species:
EDTA, DTPA, NTA, Citrate, Tris, siderophores, cobalophores,
DFB, TETA, and the famous unknown ligand(s) “L”
6
FROM LAST WEEK:
Definitions
• Ligand – an atom, ion, or
molecule that donates/shares
electrons with one or more
central atoms or ions.
• Chelate – (from Greek chelos =
crab, with two binding claws) two
or more donor atoms from a
single ligand to the central metal
atom
7
Conditional stability constants: specific to “conditions”
Thermodynamic constant
based on activities
Activity corrected,
Now based on concentrations
L- can interact with other ions:
Na+ K+ Ca2+ Mg2+
But we may not know anything
about their equilbrium constants
L- will have acid base chemistry
In seawater where there are many salts: Kcond = Kapp
If acid-base chemistry dominates: Kcond = Keff
8
We’ve already talked about the effects of salts
Acid base chemistry also matters for complexation chemistry in seawater:
We just usually don’t know enough to correctly parameterize it
experimental
modeling
Protonation constants of EDTA matter
Co2+ + 2HDMG CoHDMG2
Co2+ + EDTA4- CoEDTA2-
9
Which brings us to:
How do we measure metal speciation?
•
Use ligand exchange reactions:
Natural Ligands:
CoL Co2+ + L2-
Co2+
Our “Probe” Ligand
+ 2HDMG CoHDMG2
Net reaction:
CoL + 2HDMG CoHDMG2 + L2Core Idea: There are reactions compounds we can measure
extremely sensitively in seawater using electrochemistry
They are Electroactive like CoHDMG2
There are many electroactive ligands (synthetic):
Fe: 1N,2N; TAC,
Cu: Bzac
Zn: APDC
10
Ligand Exchange
M + L1 ML1
M + L2 ML2
ML1 + L2 ML2 +L1
11
Ligand Exchange
M + L1 ML1
M + L2 ML2
ML1 + L2 ML2 +L1
There are kinetic considerations to this:
If in seawater and either L1 or L2 has a high affinity for Ca or Mg,
it will clog up the exchange reactions
Disjunctive
ML M + L
M* + L M*L
Adjunctive
M* + ML M*LM
M*LM M*L + M
If M = Ca and M* = a trace metal the concentration gradient
is many orders of magnitude!
12
13
Trace Metal Speciation Calculations:
• Inorganic speciation Terminology:
– M’ or METAL-“PRIME” = summation of inorganic species
• Organic speciation
– L for unknown organic ligand (variants L1 and L2), metal-specific (?)
– EDTA as a “model” ligand Ethylene diaminetetraacetic acid
14
15
16
The calculation of equilibrium between multiple chemical species
Start with a simple system 3 species: M2+, MA, MB2
M + A MA
K =[MA] / [M][A]
MA = K[M][A]
M + 2B MB2
K = [MB2] / [M][B]2
MB2 = K[M][B]2
Total M = M2+ + MA + MB
Total M = M2+(1 + K[A] + K[B]2)
M2+/Total M = 1 / (1 + K[A] + K[B]2)
MA/Total M = K[A] / (1 + K[A] + K[B]2)
17
Species dependent on pH:
[CoOH-] / [Co2+][OH-] = 104.3
[H+][OH-] = 10-14
At pH 8.0: [OH-] = 10-14 / 10-8 = 10-6
[CoOH-] = 104.3[Co2+]10-6
= 10-1.7 [Co2+]
Also carbonate species, H2CO3, HCO3-, CO32- are pH dependent
and can be ligands. Acidity constants: Ka1=6.3, Ka2=10.3
[CO32-] = [CO32-]Total / ( 1 + 1010.3[H+]+1016.6[H+]2)
We typically do not assume redox equilibrium in chemical speciation
18
reactions – instead we investigate/calculate only one redox state (Fe III)
FROM LAST WEEK:
19
From Morel and Hering, 1993
20
FROM LAST WEEK:
Background Aquatic Chemistry of Trace Elements:
A marine water column context
However, there can be Non-Ideal effects:
- The effects of other solutes on the free energy of ion(s) of interest
- Solubility product and stability constants need to be corrected, or
better, determined to/at the appropriate ionic strength.
- The activity of the metal is: {Mn+} = [Mn+]gMn+
-
The activity coefficient, gMn+, can be estimated by the Debye-Huckel
correction or the Davies expression (modified Debye-Huckel)
-
Thermodynamic databases (Martell and Smith) will provide the ionic
strength experimental conditions for each constant (e.g. 0.1M)
21
22
Calculations of organic speciation in seawater
23
•
From Bruland 1988
24
Note of caution:
•
•
•
•
Tables in Morel and Hering and Stumm and Morgan are made for teaching
They have been back corrected to zero ionic strength from constants
If your application really matters, go to the literature or NIST databases for
each constant
You can use the textbooks as guidelines of species to look for though
25
History of Metal Speciation in Seawater
(Brief and Incomplete)
•
•
•
•
Cu - Sunda 1983, Coale and Bruland 1988, Moffett et al., 1990
Zn - Bruland, 1988
Cd - Bruland, 1988
Fe – Gledhill and van den Berg 1994
– Rue and Bruland 1995, Wu and Luther 1995, van den Berg 1995
• Co – Saito and Moffett 2001, Ellwood and van den Berg 2001
• Ni and Cr – Achterburg and van den Berg, 1997
• Hg – Lamborg et al., 2004
26
27
Morel, Allen, Saito, Treatise on Geochemisrty 2003
28