Balancing Chemical Equations
advertisement

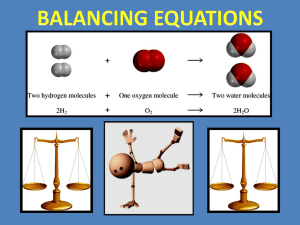
Introduction to: Balancing Chemical Equations Outline Information in Chemical Equations Why do we have to balance equations?? Human Balancing Act Balancing Chemical Equations - Easy as 1,2,3...4! Practice makes perfect or something along those lines... BIG IDEA of Unit Chemicals react with each other in predictable ways! Chocolate Chip Oatmeal Cookies Turn on oven to 170 degrees C Place first 5 ingredients in mixing bowl, mixing well after each addition In cup, dissolve baking soda and hot water, then add in mixing bowl Add flour, oats, & chocolate chips. Mix well Drop in spoonfuls onto cookie sheets Bake in oven until starting to turn golden. Makes about 50 cookies Ingredients: 1 cup butter at room temperature 1 1/2 cups brown sugar Balanced Chemical Equation 2 H2 (g) + O2 (g) → 2H2O (g) Comparison Information Communicated Recipe Chemical Equation Starting materials Ingredient list Reactants Conditions of starting materials Directions (Ie. butter at room temp) State symbols (g), (s), (l), (aq) Proportions of starting materials Quantities in ingredients list (Ie. 1 cup) Coefficients of reactants (Eg. 2H2(g)) Plus sign between formulas of Directions (Ie. mixing well after each Instructions for combining materials reactants, indicating they come into addition) contact Resulting product Title (Ie. Chocolate Chip Oatmeal Cookies) Products Proportions/Quantities of Products Final sentence (Ie. 50 cookies) Coefficients of products (Ie. 2H2O (g)) Recall (from earlier this class!): Law of conservation of mass In a given chemical equation, the mass of the reactants is equal to the mass of the products Chemical equations obey this law - show all the atoms of the reactants are still present in the products Coefficients are added before chemical formulas in an equation to ensure that the number of atoms on each side of the arrow are equal (balanced!) Human Balancing Act 1 Word equation: sodium + chlorine → sodium chloride What are the chemical formulas of the reactants and product in this equation? Human Balancing Act 1 Na + Cl2 → NaCl Volunteers needed to balance this equation! Human Balancing Act 1 Balanced Equation: 2Na + Cl2 → 2NaCl Human Balancing Act 2 A little trickier! Word equation: zinc + silver nitrate → zinc nitrate + silver What are the chemical formulas of the reactants and products in this equation?? Human Balancing Act 2 Zn + AgNO3 → ZnNO3 + Ag Volunteers to balance equation! Hint: Because polyatomic ions generally stay intact, you can count them the same way as you count atoms! Human Balancing Act Balanced Equation: Zn + 2AgNO3 → Zn(NO3)2 + 2Ag Steps for Balancing Chemical Equations: EXAMPLE: Write the balanced chemical reaction of magnesium with oxygen. STEP 1: Write word equation for the reaction Eg. magnesium + oxygen → magnesium oxide Steps for Balancing (Continued) STEP 2: Replace each chemical name with the correct chemical formula. (This is called the skeleton equation) Eg. Mg + O2 → MgO Steps for Balancing (Continued) STEP 3: Count the number of atoms of each type on either side of the arrow. Eg. Mg + O2 → MgO 1 Mg atom 1 Mg atom 2 O atoms 1 O atom Steps for Balancing (Continued) STEP 4: Multiply the formulas by an appropriate coefficient until all the atoms are balanced. Keep checking whether the numbers of each type of atom on both sides are balanced. Eg. MgO (on right) must be multiplied by coefficient 2 to balance oxygen atoms Mg + O2 → 2MgO Mg (on left) must be multiplied by coefficient 2 so there are two Mg atoms on each side 2Mg + O2 → 2MgO Balanced Chemical Equation The final balanced chemical equation is: 2Mg + O2 → 2MgO Something a little more complicated... Write a balanced chemical equation for the reaction between iron (111) nitrate and sodium hydroxide to produce iron (111) hydroxide and sodium nitrate. Step-by-Step STEP 1: iron (111) nitrate + sodium hydroxide → iron (111) hydroxide + sodium nitrate STEP 2: Fe(NO3)3 + NaOH → Fe(OH)3 + NaNO3 STEP 3: Reactants: 1 Fe atom, 3 NO3 - ions, 1 Na atom, 1 OH - ions Products: 1 Fe atom, 1 NO3- ion, 1 Na atom, 3 OH - ions Ta-da! STEP 4: Multiply NaOH (reactants) by 3 and NaNO3 (products) by 3 to get final balanced equation Fe(NO3 ) + 3NaOH → Fe(OH)3 + 3NaOH Remember... Or at least the key to chemical equations... Balancing Chemical Equations Summary: STEP 1: The word equation STEP 2: The skeleton equation STEP 3: Count atoms and ions on reactants and products side. Add coefficients! STEP 4: The balanced equation! For more assistance, consult pages 233-236 in text! Time to Practice! Worksheet on balancing chemical equations!