Chapter 6 Section 6.1
advertisement
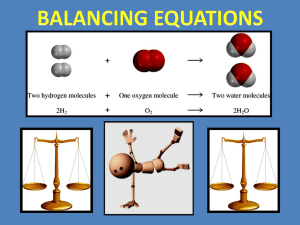
Section 6.1: Chemical Equations Objectives: Relate chemical changes and macroscopic properties, demonstrate how chemical equations describe chemical reactions, Illustrate how to balance chemical reactions by changing coefficients Identify the changes that take place when a cake is made: When a substance undergoes a chemical change After a chemical reacts, it no longer has the same chemical identity A precipitate (solid) forms Heat is produced or absorbed Odor changes Gas is given off Color change and bubbles given off indicates a good chance a chemical change has occurred, but they are not proof positive. growth of a tree melting butter the use of food in the body CO2 escaping from a can of soda separation of the components of crude oil through distillation ◦ a lake freezing ◦ ◦ ◦ ◦ ◦ Chemical Reaction (RXN): a process by which one or more substances are changed into one or more different substances; represented by an equation. Chemical Equation (EQN): shows what change takes place, and shows the relative amounts of various elements/compounds that take part in these changes. REACTANT: Substance that undergoes a reaction (change) PRODUCT: New substance formed Example: Iron and oxygen (reactants) → iron (III) oxide (Fe3+) (O2) (Fe2O3) Reactions can involve single/ or many reactants or products Reactants are placed to the left of an arrow and products are placed to the right Plus signs are used to separate reactants and also to separate products Example: hydrochloric acid + sodium hydroxide → water + sodium chloride (reactants) (products) Word equations can be converted into chemical equations Chemical formulas replace the names of compounds or elements Symbols in parentheses are put after formulas to indicate the state of the substance (s) = solid (l) = liquid (g) = gas (aq) = aqueous Example: HCl(l) + NaOH(s) → H2O(l) + NaCl(aq) Energy is often released or absorbed during chemical reactions If energy is absorbed → endothermic reaction If energy is released → exothermic reaction For reactions that absorb (need) energy, the word energy is often written along with the reactants in a chemical equation Example: 2H2O(l) + energy → 2H2(g) + O2(g) For reactions where energy is released, the word energy is often written along with the products in a chemical equation Example: CH4(g) + 2O2(g) → CO2(g) + 2H20(g) + energy Note: The word energy is not always written. If it is important to know if energy is absorbed or released or in some cases where the reaction would not occur without the addition of energy The same amount of matter is contained in the products and reactants (matter is neither created nor destroyed) Mass of reactants = Mass or products In chemical reactions, atoms do NOT change, they rearrange! The number and kinds of atoms present in the reactants of the chemical reaction are the same as those present in the products Counting atoms: The subscript after the symbol for an element represents how many atoms of that element are present For a chemical equation to accurately represent a reaction, the same number of each kind of atom must be on the left side of the arrow as are on the right side → It is said to be balanced To represent more than one unit taking part or being formed in a reaction, use a COEFFICIENT: a number is placed in front to indicate the number of units involved To balance equations, coefficients can be added or changed on the reactants and products Subscripts CAN’T be changed – changing subscripts changes the identity of that subs Analyze 2. Set Up 3. Solve 4. Check 1. 1. Magnesium metal and water combine to form solid magnesium hydroxide and hydrogen gas Analyze: (word equation) magnesium + water → magnesium hydroxide + hydrogen Set up: (chemical equation) Mg(s) + H2O(l) → Mg(OH)2(s) + H2(g) Solve: (count atoms and then balance) Left: Mg = 1, H = 2, O = 1 Right: Mg = 1, H= 4, O = 2 Mg(s) + 2H2O(l) → Mg(OH)2(s) + H2(g) Check: (count atoms) Left: Mg = 1, H = 4, O = 2 Right: Mg = 1, H= 4, O = 2 The equation is properly balanced. Hydrogen and oxygen combine to form gaseous water and release energy. Word equation: Hydrogen + oxygen -> water + energy Chemical equation: H2(g) + O2(g) -> H2O (g) + energy Balance: 2H2(g) + O2(g) -> 2H2O (g) + energy P. 199 (#1,3) Write word equations and chemical equations 1) Magnesium and water combine to form solid magnesium hydroxide and hydrogen gas. 3) When energy is added to solid manganese(II) sulfate heptahydrate crystals, they break down to form liquid water and solid manganese(II) sulfate monohydrate P. 199 (#2,4) Write word equations and chemical equations 2) An aq. Solution of dihydrogen dioxide and solid lead (II) sulfide combine to form solid lead (II) sulfate and liquid water. 4) Solid potassium reacts with liquid water to produce aq. Potassium hydroxide and hydrogen gas