Osmosis Final
advertisement

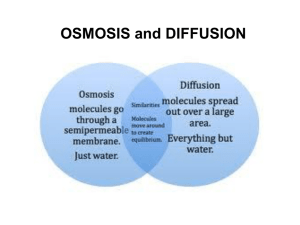
Cell Membranes Diffusion, Osmosis & Osmotic Pressure Dr. Kashif Rahim 1 Functions of Membranes 1. Protect cell 2. Control incoming and outgoing substances 3. Maintain ion concentrations of various substances 4. Selectively permeable - allows some molecules in, others are kept out Dr. Kashif Rahim 2 Phospholipid Bilayer Dr. Kashif Rahim 3 Fluid Mosaic Model Dr. Kashif Rahim 4 Solutions • Solutions are made of solute and a solvent • Solvent - the liquid into which the solute is poured and dissolved. We will use water as our solvent today. • Solute - substance that is dissolved or put into the solvent. Salt and sucrose are Dr. Kashif Rahim 5 solutes. Methods of Transport Across Membranes 1. Diffusion 2. Osmosis 3. Facilitated Diffusion 4. Active Transport Dr. Kashif Rahim 6 Methods of Transport Across Membranes 1. Diffusion -passive transport - no energy expended 2. Osmosis - Passive transport of water across membrane 3. Facilitated Diffusion - Use of proteins to carry polar molecules or ions across 4. Active Transport- requires energy to transport molecules against a concentration gradient – energy is in the form of ATP Dr. Kashif Rahim 7 Diffusion • Particles in a liquid or gas spread out… • … from regions of high concentration… • … to regions of low concentration… • …until the particles are evenly spread out. Dr. Kashif Rahim Dissolving KMnO4 crystal 8 • The difference between the regions of high concentration and low concentration is called the concentration gradient • The steeper the concentration gradient, the faster diffusion takes place High concentration gradient Fast rate of diffusion Low concentration gradient Slow rate of diffusion Dr. Kashif Rahim 9 • Diffusion occurs because the particles in gases and liquids are moving. Dr. Kashif Rahim 10 Diffusion Dr. Kashif Rahim 11 Dissolving substances in water • The molecules in liquid water are constantly moving • When water molecules bump into particles of a soluble substance, they stick to them Free moving water molecules Sugar molecules in sugar lump Dr. Kashif Rahim 12 • When the water molecules move away… … they carry particles of the solute with them Dr. Kashif Rahim 13 • Adding a solute to water reduces the amount of free water molecules Free water molecules Solute molecule Dr. Kashif Rahim 14 Partially-Permeable Membranes • A partially-permeable membrane will allow certain molecules to pass through it, but not others. Partially permeable membrane • Generally, small particles can pass through… Dr. Kashif Rahim …but large particles cannot 15 More free water molecules on this side of membrane Partially-permeable membrane Water-solute particle is too large to pass through membrane Free water molecules diffuse in this direction Dr. Kashif Rahim 16 Osmosis • Osmosis is the diffusion of free water molecules… •… from a region of high concentration of free water molecules… • … to a region of low concentration of free water molecules… • …across a partially-permeable membrane… • …until they are evenly spread out. Dr. Kashif Rahim 17 Distilled water separated by a partiallypermeable membrane: Water molecules are moving from one side of the membrane to the other but there is no net osmosis Dr. Kashif Rahim 18 If a substance is dissolved in water, the kinetic energy of the water molecules is lowered. This is because some water molecules aggregate on the surfaces of the other molecules… Dr. Kashif Rahim 19 Tonicity is a relative term • Hypotonic Solution - One solution has a lower concentration of solute than another. • Hypertonic Solution - one solution has a higher concentration of solute than another. • Isotonic Solution - both solutions have same concentrations of solute. Dr. Kashif Rahim 20 For osmosis we talk about the potential water molecules have to move – the OSMOTIC POTENTIAL. Distilled water has the highest potential (zero). When water has another substance dissolved in it, the water molecules have less potential to move. The osmotic potential is NEGATIVE. Dr. Kashif Rahim 21 Water molecules always move from less negative to more negative water potential. Net osmosis = LN MN Dr. Kashif Rahim 22 The osmotic potential of a cell is known as its WATER POTENTIAL. For animal cells, the water potential is the osmotic potential of the cytoplasm. Dr. Kashif Rahim 23 An animal cell with water potential –50 is placed in a solution… Dr. Kashif Rahim 24 Water potential of cytoplasm = -50 Osmotic potential of solution= -20 Dr. Kashif Rahim If the osmotic potential of the solution is less negative than the water potential of the cytoplasm(the solution is hypotonic), net endosmosis will occur, i.e. water will move into the cell from the solution. The result will be haemolysis (the cell will burst) 25 Water potential of cytoplasm= -50 Osmotic potential of solution = -80 Dr. Kashif Rahim If the osmotic potential of the solution is more negative than the water potential of the cytoplasm (the solution is hypertonic), net exosmosis will occur. The result will be crenation (the cell will shrivel up) 26 Water potential of cytoplasm= -50 Osmotic potential of solution= -50 Dr. Kashif Rahim If the osmotic potential of the solution is the same as the water potential of the cytoplasm (the solution is isotonic), there will be no net osmosis. 27 What controls osmosis? • Unequal distribution of particles, called a concentration gradient, is one factor that controls osmosis. Before Osmosis Selectively permeable membrane Dr. Kashif Rahim After Osmosis Water molecule Sugar molecule 28 Osmosis: Diffusion of Water • Most cells whether in multicellular or unicellular organisms, are subject to osmosis because they are surrounded by water solutions. Dr. Kashif Rahim 29 Cells in an isotonic solution • isotonic solution• (= concentrations) • the concentration of dissolved substances in the solution is the same as the concentration of dissolved substances inside the cell. H2O H2O Water Molecule Dissolved Molecule Dr. Kashif Rahim 30 Cells in an isotonic solution H2O • water molecules move into and out of the cell at the same rate, and cells retain their normal shape. H2O Water Molecule Dissolved Molecule Dr. Kashif Rahim 31 Cells in a hypotonic solution • hypotonic solution: dilute solution thus low solute concentration • In a hypotonic solution, water enters a cell by osmosis, causing the cell to swell. H2O H2O Water Molecule Dissolved Molecule Dr. Kashif Rahim 32 Cells in a hypertonic solution • hypertonic solution: concentrated solution, thus a high solute concentration In a hypertonic solution, water leaves a cell by osmosis, causing the cell to shrink H2O H2O Water Molecule Dissolved Molecule Dr. Kashif Rahim 33 Osmosis Dr. Kashif Rahim 34 Semi permeable Membrane Theory of Osmosis Pressure Fresh Water Fresh Water H2O Sea Water H2O Sea Water (diluted) H2O Fresh Water Sea Water H2O Initial Condition Equilibrium Reverse Osmosis The Osmotic Pressure, π, is defined as: π = MRT For sea water at Dr. 35Kashif ppt, Rahim π is about 350 psi. 35 Reverse Osmosis is a water treatment process whereby dissolved salts, such as sodium, chloride, calcium carbonate, and calcium sulfate may be separated from water by forcing the water through a semi-permeable membrane under high pressure. The water diffuses through the membrane and the dissolved salts remain behind on the Dr. Kashif Rahim 36 surface of the membrane. Osmotic Pressure • For the phenomenon of osmosis, a membrane separates salt/water inside a chamber from pure water in the container. Water passes through membrane from dilute to more concentrated. As water rises into tube, it creates a pressure. Eventually this pressure (osmotic pressure) prevents further passage of water through the membrane • Osmotic pressure is force per area that prevents water from passing through membrane! Dr. Kashif Rahim 37 Dr. Kashif Rahim 38 Diagram of osmotic pressure cell. Dr. Kashif Rahim 39 Osmotic Pressure • Osmotic pressure. A semi permeable membrane allows water molecules, but not salt ions or large molecules, to pass by osmosis unless osmotic pressure is applied. The osmotic pressure cell pictured here allows water molecules to pass from the low ion concentration side (left) through the semi permeable membrane to the higher ion concentration (right). The pressure gauge shows the increase in pressure caused by the water Dr. Kashif Rahim 40 Osmotic Pressure • Methods for the determination of osmotic pressure are 1. Pfeffer’s method 2. Freezing point determination method. Decrease in freezing point of the solution when its osmotic pressure is equal to one osmole. Dr. Kashif Rahim 41 Osmotic Pressure • Laws of Osmotic Pressure : 1. The Osmotic Pressure is directly proportional to the concentration of the solute. 2. The Osmotic Pressure is directly proportional to the absolute temperature. Dr. Kashif Rahim 42 Osmotic Pressure • Importance of osmotic pressure of plasma proteins: 1. The plasma proteins form a colloidal solution and are the chief colloid of the plasma. 2. The oncotic pressure of the plasma proteins is the main force which tends to keep the plasma water within the blood vessels. 3. If concentration of plasma proteins decreases, water will leak into tissue spaces and will lead to development of edema. Dr. Kashif Rahim 43 Dr. Kashif Rahim 44