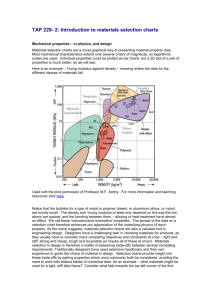
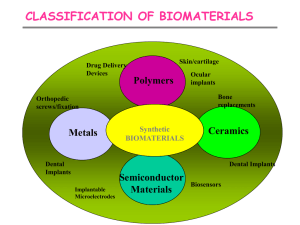
Ceramics (Chapter 11 – reference)
advertisement
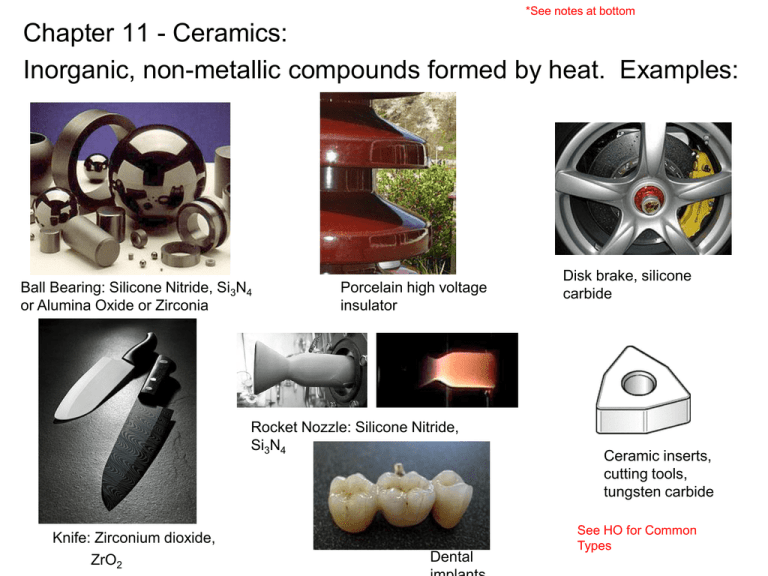
*See notes at bottom Chapter 11 - Ceramics: Inorganic, non-metallic compounds formed by heat. Examples: Ball Bearing: Silicone Nitride, Si3N4 or Alumina Oxide or Zirconia Porcelain high voltage insulator Rocket Nozzle: Silicone Nitride, Si3N4 Knife: Zirconium dioxide, ZrO2 Dental Disk brake, silicone carbide Ceramic inserts, cutting tools, tungsten carbide See HO for Common Types III. Types of Ceramics: I. Overview of ceramics: Characterized by: 1. Compounds between metallic and non-metallic elements (i.e. Si and O, Si and N, Al and O, etc..) 2. Frequently oxides, nitrides and carbides (i.e. silicon carbide – SpinWorks) 3. Can be crystalline or amorphous 4. Very strong covalent (sharing of electrons) or ionic (transfer of electrons) bonds. 5. Properties include: • Strong but brittle • Low fracture toughness • Good insulators of electricity BUT good conductor of heat (i.e. comparable to metals have reasonably high thermal conductivity, k) – this is unique to ceramics. • Excellent high temp properties • Low coef of thermal expansion I. Nature of ceramics: Example: Aluminum Oxide, Al2O3 Covalent = strong Electrons tied up II. Properties of Ceramics • Benefits: – High chemical resistance – High melting point and therefore high operating T. – Extremely hard and stiff (i.e. 180 E6 psi) – Good electrical insulator (electrons tied up). Exception = superconductors – Good thermal conductor (high K like metals) – But, low thermal expansion and good thermal stability – Good creep – High modulus – High compressive strength II. Properties of Ceramics • Shortcomings: – Low tensile strength and BRITTLE. Sut = Suc/10. Do not readily slip like metals but fracture. – Catastrophic failure • The bond is ionic and covalent. A material held by either type of bond will tend to fracture before any plastic deformation can occur! – Low fracture toughness (1/10 to 1/100 KIC compared to metals) • Materials tend to be porous and microscopic imperfections act as stress concentration decreasing the toughness further. – Elongation = 0% – Low fatigue strength – Large statistical spread and less predictable than metals (size, shape and location of internal flaws is likely to differ from part to part) – Prone to thermal shock – Hard to machine and form – Cost 8X more than metals – See Table 8.2 Optimized selection using charts Search area 1 E M ρ 2 3 Results E1/2 ρ M E1/3 M ρ 22 pass Material 1 Material 2 Material 3 etc... Ranked by Index E1/2 /ρ 2230 2100 1950 Electrical Resistance: Ceramics = good electrical insulators, but… Selection: one-property indices Good thermal conductors! Good conductors: metals and ceramics Good insulators: polymer foams, cork, wood, cardboard…. III. Types of Ceramics: (see HO 6 – 10): a) Structural (clay) and whiteware: bricks, pipes, floor and roof tiles, dinner ware, chinca, etc… b) Refractory ceramics: kiln lingings, high T capability most are based on silicates (sand) c) Glass, amorphous ceramic, most based on silica, SiO2 – Annealed glass Go to HO pg 6!! – Tempered glass – Laminated glass III. Types of Ceramics: d) (see HO 6 – 10): Technical or engineering ceramics – 3 categories: i. Oxides (alumina, zirconia) semiconductors ii. Non-oxide (carbides, borides, nitrides) i.e. tungsten carbide cutting tools, silicone nitride ball bearings, silicone carbide furnace inserts. iii. Cermits or composites – combination of metals and ceramics (powder metallurgy), combines high strength and hardness, thermal characteristic of ceramics with toughness of metals) V. How to Strengthen: (see HO 4,5): a) Flame polishing to reduce surface cracks b) Close surface cracks use in compression (or compress with metal band) c) Atom gun to fill in surface cracks (fires ions into cracks) d) Reduce crystal size e) Laminate or anneal (glass) f) Combine materials to increase toughness (cermits) V. Manufacturing – see HO • In situ – cement – mix powder with water • Sintering based methods WATCH!!!! http://www.youtube.com/watch?v=69Y0VuOYqkU http://ceramicinjectionmolding.com/