Nervous System - Dr. Eric Schwartz
advertisement

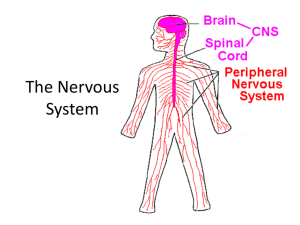
Chapter 06 Lecture Outline* Neuronal Signaling and the Structure of the Nervous System Eric P. Widmaier Boston University Hershel Raff Medical College of Wisconsin Kevin T. Strang University of Wisconsin - Madison *See PowerPoint Image Slides for all figures and tables pre-inserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 The Nervous System • The Nervous System has two major divisions: – The Central Nervous System (CNS), which is composed of the brain and spinal cord. – The Peripheral Nervous System (PNS) is composed of the nerves that connect the brain or spinal cord with the body’s muscles, glands, and sense organs. • The neuron is the basic cell type of both systems. 2 Structure of a Neuron Fig. 6-1 3 Neurons • These are the “nerve cells”. • They are amitotic, so they do not divide. They also have a very high metabolic rate. • Clusters of cell bodies in the CNS are called nuclei. • Neurons are not the most numerous cell in the CNS; Glial cells are. 4 Glial Cells Fig. 6-6 5 Glial Cells of the CNS • The glial cells in the CNS are: • Astrocytes: support cells, control extracellular environment of neurons • Microglia:”immune system” of the CNS • Ependymal cells: ciliated, involved with production of CSF and CSF movement • Oligodendrocytes: responsible for the myelin 6 Glial Cells of the PNS • The PNS glial cells are the satellite cells and the Schwann cells. • Satellite cells surround neuron bodies located in the PNS. • Schwann cells surround and form myelin sheaths around the larger nerve fibers. These are vital to regeneration and proper nerve signal conduction. 7 Schwann Cells & Myelin Fig. 6-2 8 Axonal Transport Maintains Axon Structure & Function Fig. 6-3 9 Functional Classes of Neurons Fig. 6-4 10 11 Development of the Nervous System • Development of the nervous system in the embryo begins with stem cells that can develop into neurons or glial cells. • After the last cell division, each neuronal daughter cell differentiates, migrates to its final location, and sends out processes that will become its axon and dendrites. • A specialized enlargement, the growth cone, forms the tip of each extending axon and is involved in finding the correct route and final target for the process. • As the axon grows, it is guided along the surfaces of other cells, most commonly glial cells. 12 Development of the Nervous System • Which route the axon follows depends largely on attracting, supporting, deflecting, or inhibiting influences exerted by cell adhesion molecules and soluble neurotrophic factors (growth factors for neural tissue) in the extracellular fluid surrounding the growth cone or its distant target. • Once the target of the advancing growth cone is reached, synapses form. • During these early stages of neural development, which occur during all trimesters of pregnancy and into infancy, alcohol and other drugs, radiation, malnutrition, and viruses can exert effects that cause permanent damage to the developing fetal nervous system. 13 Development of the Nervous System • Early in development, the brain has much greater potential for remodeling in response to stimulation or injury than in the adult brain, a characteristic known as plasticity. • The basic shapes and locations of major neuronal circuits in the mature central nervous system do not change once formed. • The creation and removal of synaptic contacts begun during fetal development continue, however, though at a slow pace throughout life as part of normal growth, learning, and aging. 14 Injury of the Nervous System • If axons are severed, they can repair themselves and restore significant function provided that the damage occurs outside the central nervous system and does not affect the neuron’s cell body. • After such an injury, the axon segment that is separated from the cell body degenerates. The part of the axon still attached to the cell body then gives rise to a growth cone, which grows out to the effector organ so that function is sometimes restored. • Return of function following a peripheral nerve injury is delayed because axon regrowth proceeds at a rate of only 1 mm per day. 15 Injury of the Nervous System • Spinal injuries typically crush rather than cut the tissue, leaving the axons intact. • In this case, the problem is apoptosis of the oligodendrocytes. This results in loss of the myelin coat and the axons cannot transmit information effectively. • Severed axons within the CNS may grow small new extensions but no significant regeneration of the axon occurs across the damaged site, and there are no well-documented reports of significant return of function. 16 New Attempts to Repair Nervous System Damage • Researchers are trying a variety of ways to provide an environment that will support axonal regeneration in the central nervous system. – They are creating tubes to support regrowth of the severed axons, redirecting the axons to regions of the spinal cord that lack growth-inhibiting factors, preventing apoptosis of the oligodendrocytes so myelin can be maintained, and supplying neurotrophic factors that support recovery of the damaged tissue. • Medical researchers are also attempting to restore function to damaged or diseased brains by implanting stem cells, pieces of fetal brain or other brain tissues to replace the lost functions. 17 Synapses Synapses can use both chemical and electrical stimuli to pass information. Synapses can also be inhibitory or excitatory depending on the signal/ neurotransmitter being transmitted. Fig. 6-5 18 Basic Principles of Electricity Fig. 6-7 19 The Resting Membrane Potential Fig. 6-8 20 21 Membrane Potentials • Different cells have different resting membrane potentials. Neurons have a resting membrane potential generally in the range of –40 to –90 mV. • Changes in potential are due to movement of ions. • We can calculate the contributions of individual ions with the Goldman-Hodgkin-Katz (GHK) equation: The GHK equation is essentially an expanded version of the Nernst equation that takes into account individual ion permeabilities. 22 Establishing Membrane Potential • First, the action of the Na+/K+-ATPase pump sets up the concentration gradients for Na+ and K+ (Figure 6–13a). • Then there is a greater flux of K+ out of the cell than Na+ into the cell (Figure 6–13b). This is because in a resting membrane there are a greater number of open K+ channels than there are Na+ channels. Because there is greater net efflux than influx of positive ions during this step, a significant negative membrane potential develops, with the value approaching that of the K+ equilibrium potential. • In the steady-state, the flux of ions across the membrane reaches a dynamic balance (Figure 6–13c). Because the membrane potential is not equal to the equilibrium potential for either ion, there is a small but steady leak of Na+ into the cell and K+ out of the cell. • The concentration gradients do not dissipate over time, however, because ion movement by the Na+/K+-ATPase pump exactly balances the rate at which the ions leak through open channels. 23 Establishing the Resting Membrane Potential Fig. 6-13 24 Terminology • When talking about action potentials and graded potentials we use these terms: depolarization, repolarization, hyperpolarization. • These terms are all relative to the resting membrane potential (RMP). • Depolarization is the potential moving from RMP to less negative values. • Repolarization is the potential moving back to the RMP. • Hyperpolarization is the potential moving away from the RMP in a more negative direction. 25 Fig. 6-14 26 Depolarization Fig. 6-15 27 Graded Potentials • Graded potentials are changes in membrane potential that are confined to a relatively small region of the plasma membrane. • They are called graded potentials simply because the magnitude of the potential change can vary (is “graded”). • Graded potentials are given various names related to the location of the potential or the function they perform; for instance, receptor potential, synaptic potential, and pacemaker potential. 28 Graded Potentials Fig. 6-16 29 Action Potentials • Action potentials are generally very rapid (as brief as 1–4 milliseconds) and may repeat at frequencies of several hundred per second. • The ability to generate action potentials is known as excitability. This ability is possessed by neurons, muscle cells and some other types of cells. • An action potential is a large change in membrane potential and is an “all or none” response. 30 Action Potential Membrane Depolarization • In order to cause an action potential, a cell must utilize several types of ion channels. • Ligand-gated channels and mechanically gated channels often serve as the initial stimulus for an action potential. • The voltage-gated channels give a membrane the ability to undergo action potentials by allowing the rapid depolarization and repolarization phases of the response. • There are dozens of different types of voltage-gated ion channels, varying by which ion they conduct (e.g., Na+, K+, Ca2+, or Cl-) and in how they behave as the membrane voltage changes. 31 Voltage-Gated Na+ & K+ Channels Fig. 6-18 32 Mechanism of an Action Potential Fig. 6-19 33 Control Mechanisms of an Action Potential Fig. 6-20 34 Clinical Effects of Action Potential Inhibition • The generation of action potentials is prevented by local anesthetics such as procaine (Novocaine®) and lidocaine (Xylocaine®) because these drugs block voltage-gated Na+ channels. • Without action potentials, graded signals generated in the periphery—in response to injury, for example—cannot reach the brain and give rise to the sensation of pain. • Some animals produce toxins that work by interfering with nerve conduction in the same way that local anesthetics do. For example, the puffer fish produces tetrodotoxin, that block voltage-gated Na+ channels. 35 Refractory Period • There are 2 types of refractory periods that cells undergo following an action potential: Absolute and Relative. • The absolute refractory period is during the action potential; a second stimulus, no matter how strong, will not produce a second action potential . • This occurs during the period when the voltage-gated Na+ channels are either already open or have proceeded to the inactivated state during the first action potential. • Following the absolute refractory period, there is an interval during which a second action potential can be produced, but only if the stimulus strength is considerably greater than usual. 36 Refractory Period • The refractory periods limit the number of action potentials an excitable membrane can produce in a given period of time. • Most neurons respond at frequencies of up to 100 action potentials per second, and some may produce much higher frequencies for brief periods. • Refractory periods contribute to the separation of these action potentials so that individual electrical signals pass down the axon. • The refractory periods also are the key in determining the direction of action potential propagation. 37 Refractory Period Fig. 6-22 38 Action Potential Propagation • Action potentials in neurons are unidirectional (can only go forward down the axon, since the space behind is in its refractory period). • In skeletal muscle cells the action potentials are initiated near the middle of the cells and propagate toward the two ends. • The velocity with which an action potential propagates along a membrane depends upon fiber diameter and whether or not the fiber is myelinated. • The larger the fiber diameter, the faster the action potential propagates, because a large fiber offers less resistance to local current; more ions will flow in a given time. 39 Action Potential Propagation • Myelin is an insulator that makes it more difficult for charge to flow between intracellular and extracellular fluid compartments. • Action potentials occur only at the nodes of Ranvier, where the myelin coating is interrupted and the concentration of voltage-gated Na+ channels is high. • Thus, action potentials jump from one node to the next as they propagate along a myelinated fiber, and for this reason such propagation is called saltatory conduction. 40 Action Potential Propagation • Propagation via saltatory conduction is faster than propagation in nonmyelinated fibers of the same axon diameter. • Moreover, because ions cross the membrane only at the nodes of Ranvier, the membrane pumps need to restore fewer ions. • Myelinated axons are metabolically more efficient than unmyelinated ones. • Myelin adds speed, reduces metabolic cost, and saves room in the nervous system because the axons can be thinner. 41 Action Potential Propagation Fig. 6-23 42 Saltatory Conduction Fig. 6-24 43 44 Synapses Fig. 6-5 45 Synapses • Synapses are junctions between two neurons. • They can be chemical or electrical. • In an electrical synapse, the electrical activity of the presynaptic neruon affects the electrical activity of the postsynaptic neuron. • Chemical synapses utilize neurotransmitters. 46 Functional Anatomy of Synapses • Electrical – Pre- and post-synaptic cells are connected by gap junctions • Chemical – Pre-synaptic neurons release neurotransmitter from their axon terminals – Neurotransmitter binds to receptors on postsynaptic neurons 47 Anatomy of a Chemical Synapse Fig. 6-26A 48 Mechanisms of Neurotransmitter Release Fig. 6-27 49 Docking of Vesicles and Release of Neurotransmitters • Neurotransmitters are produced and stored in vesicles at the axon terminal. • When the cell is stimulated the intracellular Ca2+ levels increase and stimulate the vesicles to translocate and bind to the plasma membrane via the SNARE proteins. • The neurotransmitter is then released via exocytosis. 50 Removal of Neurotransmitter • To terminate the signal in a chemical synapse the neurotransmitter must be removed. This is accomplished by: 1. Diffusion of the transmitter from the cleft 2. Degradation of the transmitter by enzymes 3. Reuptake into the pre-synaptic cells for reuse • Receptors in the post-synaptic cell are removed from the membrane. 51 Activation of the Postsynaptic Cell • Excitatory chemical synapses generate an excitatory postsynaptic potential (EPSP). • EPSPs serve to bring the membrane potential closer to threshold for generating an action potential. • Inhibitor chemical synapses generate an inhibitory postsynaptic potential (IPSP). • IPSPs make the cell’s membrane potential more negative, making it harder to generate an action potential. 52 Excitatory Postsynaptic Potential Fig. 6-28 53 Inhibitory Postsynaptic Potential Fig. 6-29 54 Synaptic Integration Fig. 6-31 55 Autoreceptors Autoreceptors are a built-in brake for the system. Once the neurotransmitter is released it will diffuse and activate the post-synaptic cell, but also bind to the autoreceptors and turn off further release from the pre-synaptic cell. Fig. 6-33 56 57 Modification of Synaptic Transmission by Drugs and Disease • Drugs act by interfering with or stimulating normal processes in the neuron involved in neurotransmitter synthesis, storage, and release, and in receptor activation. • Diseases can also affect synaptic mechanisms. Examples: Clostridium tetani (tetanus toxin) prevents vesicle fusion with the membrane, inhibiting neurotransmitter release and causes increased muscle contraction. Clostridium botulinum bacilli toxin (botulism), interferes with actions of SNARE proteins at excitatory synapses that activate muscles; botulism is characterized by muscle paralysis. Low doses of botulinum toxin (Botox) are injected therapeutically to treat a number of conditions, including facial wrinkles, severe sweating, hypercontracted neck muscles, uncontrollable blinking, misalignment of the eyes, and others. 58 59 Neuromodulators • Neuromodulators modify both the presynaptic and the postsynaptic cell’s response to specific neurotransmitters, amplifying or dampening the effectiveness of ongoing synaptic activity. • Receptors for neurotransmitters affect ion channels that directly affect excitation or inhibition of the postsynaptic cell, and these mechanisms operate within milliseconds. • Receptors for neuromodulators bring about changes in metabolic processes in neurons, and these changes can occur over minutes, hours, or even days, include alterations in enzyme activity or, through influences on DNA transcription, in protein synthesis. • Thus, neurotransmitters are involved in rapid communication, whereas neuromodulators tend to be associated with slower events such as learning, development, motivational states, and some types of sensory or motor activities. 60 Acetylcholine • Acetylcholine (ACh) is found in PNS and CNS. Neurons that use ACh as the primary neurotransmitter are known as cholinergic neurons. • ACh acts at muscarinic (G protein coupled) or nicotinic (ion channels) receptors. Nicotininic receptors are found at the neuromuscular junctions of skeletal muscles. • ACh is produced in the presynaptic axon by the enzyme choline acetyl transferase (CAT) as follows: • Acetyl CoA + choline acetylcholine + CoA • Degradation of ACh occurs in synaptic cleft and is done by the enzyme acetylcholinesterase (AChE) as follows: • Acetylcholine acetate + choline 61 Fig. 6-34 62 Cholinergic System Issues • Some chemical weapons, such as the nerve gas Sarin, inhibit acetylcholinesterase, causing a buildup of ACh in the synaptic cleft. • Overstimulation of postsynaptic ACh receptors causes uncontrolled muscle contractions, ultimately leading to receptor desensitization and paralysis. • Nicotinic receptors in the brain are important in cognitive functions and behavior. The presence of nicotinic receptors on presynaptic terminals in reward pathways of the brain explains why tobacco products are among the most highly addictive substances known. 63 Alzheimer’s Disease • Neurons associated with the ACh system degenerate in people with Alzheimer’s disease. Alzheimer’s disease affects 10 to 15 percent of people over age 65, and 50 percent of people over age 85. • Because of the degeneration of cholinergic neurons, this disease is associated with a decreased amount of ACh in certain areas of the brain and even the loss of the postsynaptic neurons that would have responded to it. • These defects and those in other neurotransmitter systems that are affected in this disease are related to the declining language and perceptual abilities, confusion, and memory loss that characterize Alzheimer’s victims. 64 Biogenic Amines • Biogenic amine neurotransmitters are made from amino acids as follows: • Catecholamines • Made from tyrosine: • Dopamine • Norepinephrine • Epinephrine • Made from tryptophan: • Serotonin • Made from histidine: • Histamine • The enzymes which degrade the biogenic amine neurotransmitters are: • Monoamine oxidase (MAO) • Catechol-o-methyltransferase 65 Synthesis of Catecholamines Fig. 6-35 66 Parkinson’s Disease • This disease involves the loss of dopamine-releasing neurons in the substantia nigra. • Symptoms include persistent tremors, head nodding and pill rolling behaviors, a forward bent walking posture, shuffling gait, stiff facial expressions and they are slow in initiating and executing movement. • The cause is not clearly understood, but loss of the dopamine neurons is critical. • It is currently treated with the drug L-Dopa in the initial stages to alleviate symptoms. This is often given with the drug deprenyl (which prevents the breakdown of L-Dopa). This is NOT curative. We can only treat the symptoms. • Experimental treatments currently include deep brain stimulation by surgically implanting electrodes, gene therapy and fetal/stem cell transplants. 67 Adrenergic Receptors • Adrenergic receptors are utilized by the neurotransmitters Norepinphrine (NE) and Epinephrine (Epi). NE is found in both the CNS and PNS but Epi is found mainly in the PNS. Adrenergic comes from historical use as Noradrenaline (NA) and adrenaline for NE and Epi, respectively. • Adrenergic receptors are G protein coupled that are generally linked to second messenger signal transduction pathways. • Alpha adrenergic receptors ‒ Alpha1 (α1) ‒ Alpha2 • Beta adrenergic receptors ‒ Beta1 (β1) ‒ Beta2 ‒ Beta3 68 Serotonin • Also known as 5-hydroxytryptamine or 5-HT • CNS neurotransmitter and is also made by enterochromaffin cells in the gut and is taken up and stored in nerve terminals and platelets • Main CNS location • Brainstem • Functions • Regulating sleep • Emotions • 5-HT3 receptors in the area postremia are involved in the vomiting reflex • Regulates cell growth • Vascular smooth muscle cell contraction 69 Histamine • CNS neurotransmitter whose major location is the hypothalamus • Histamine is commonly known for paracrine actions. • Histamine is also found in the peripheral system. It is involved in allergic reactions, nerve sensitization, and acid production in the stomach. 70 Amino Acid Neurotransmitters • Amino acid neurotransmitters at excitatory synapses are: • Aspartate • Glutamate • Amino acid neurotransmitters at inhibitory synapses are: • Glycine • GABA 71 Glutamate • Glutamate is estimated to be the primary neurotransmitter at 50 percent of the excitatory synapses in the CNS. • There are 2 types of receptors: – Metabotropic glutamate receptors (G-protein Coupled receptors) – Ionotropic glutamate receptors • AMPA receptors (identified by their binding to a-amino-3 hydroxy-5 methyl-4 isoxazole proprionic acid) • NMDA receptors (which bind N-methyl-D-aspartate) 72 Glutamate • Cooperative activity of AMPA and NMDA receptors has been implicated in a phenomenon called long-term potentiation (LTP). • This mechanism couples frequent activity across a synapse with lasting changes in the strength of signaling across that synapse, and is thus thought to be a cellular process underlying learning and memory. • Figure 6–36 outlines the mechanism in stepwise fashion. 73 Glutamatergic Synapses Fig. 6-36 74 Glutamate Actions • Step 1. Presynaptic neuron fires action potentials. • Step 2. Glutamate is released from presynaptic terminals. • Step 3. Glutamate binds to both AMPA and NMDA receptors on postsynaptic membranes. • Step 4. Depolarizing EPSP of the postsynaptic cell mediated via AMPA channels (Na+). • Step 5. The depolarization through the AMPA channels allows the magnesium ion blocking the NMDA channels to move and activate the channel. NMDA-receptor channels mediate a substantial Ca2+ flux. • Step 6. Calcium enters the cell. • Step 7. Calcium ions activate second-messenger cascade in the postsynaptic cell that includes persistent activation of two different protein kinases, and which increases the sensitivity of the postsynaptic neuron to glutamate. • Step 8. This second-messenger system can also activate long-term enhancement of presynaptic glutamate release via retrograde signals that have not yet been identified. 75 NMDA Receptors • NMDA receptors have also been implicated in mediating excitotoxicity. • This is a phenomenon in which the injury or death of some brain cells (due, for example, to blocked or ruptured blood vessels) rapidly spreads to adjacent regions. • When glutamate-containing cells die and their membranes rupture, the flood of glutamate excessively stimulates AMPA and NMDA receptors on nearby neurons. • The excessive stimulation of those neurons causes the accumulation of toxic levels of intracellular Ca2+, which in turn kills those neurons and causes them to rupture, and the wave of damage progressively spreads. • Recent experiments and clinical trials suggest that administering NMDA receptor antagonists may help minimize the spread of cell death following injuries to the brain. 76 GABA • GABA (gamma-aminobutyric acid) is the major inhibitory neurotransmitter in the brain. • Although it is not one of the 20 amino acids used to build proteins, it is classified with the amino acid neurotransmitters because it is a modified form of glutamate. • GABA neurons in the brain are small interneurons that dampen activity within neural circuits. Postsynaptically, GABA may bind to ionotropic or metabotropic receptors. • The ionotropic receptor increases Cl- flux into the cell, resulting in hyperpolarization of the postsynaptic membrane. 77 GABA • In addition to the GABA binding site, this receptor has several additional binding sites for other compounds, including steroids, barbiturates, and benzodiazepines. • Benzodiazepine drugs such as alprazolam (Xanax®) and diazepam (Valium®) reduce anxiety, guard against seizures, and induce sleep, by increasing Cl- flux through the GABA receptor. • Synapses that use GABA are also among the many targets of the ethanol found in alcoholic beverages. 78 GABA and Alcohol • Ethanol stimulates GABA synapses and simultaneously inhibits excitatory glutamate synapses, with the overall effect being global depression of the electrical activity of the brain. • Thus, as a person’s blood alcohol content rises, there is a progressive reduction in overall cognitive ability, along with reduced sensory perception (hearing and balance in particular), motor incoordination, impaired judgment, memory loss, and unconsciousness. • Very high doses of ethanol are sometimes fatal, due to suppression of brainstem centers responsible for regulating the cardiovascular and respiratory systems. 79 Glycine • Glycine is the major neurotransmitter released from inhibitory interneurons in the spinal cord and brainstem. It binds to ionotropic receptors on postsynaptic cells that allow Cl- to enter. • Normal function of glycinergic neurons is essential for maintaining a balance of excitatory and inhibitory activity in spinal cord integrating centers that regulate skeletal muscle contraction. • This becomes apparent in cases of poisoning with the neurotoxin strychnine, an antagonist of glycine receptors. Victims experience hyperexcitability throughout the nervous system, which leads to convulsions, spastic contraction of skeletal muscles, and ultimately death due to impairment of the muscles of respiration. 80 Neuropeptides • Neuropeptides are short chains of amino acids with peptide bonds. Some 85 neuropeptides have been identified, but their physiological roles are not all known. • In the cell body, the precursor protein is packaged into vesicles, which are then moved by axonal transport into the terminals where the protein is cleaved by specific peptidases. • Many of the precursor proteins contain multiple peptides, which may be different or be copies of one peptide. Neurons that release one or more of the peptide neurotransmitters are collectively called peptidergic. In many cases, neuropeptides are cosecreted with another type of neurotransmitter and act as neuromodulators. 81 Neuropeptides • Examples include: • Endogenous opioids • Enkephalins • Endorphins • Morphine and codeine are synthetic opioids that are used as analgesics (pain reducers). • Substance P • Released by afferent neurons that relay sensory information into the central nervous system. • It is known to be involved in pain sensation. 82 Gas Neurotransmitters • Gases are not released by exocytosis of presynaptic vesicles, nor do they bind to postsynaptic plasma membrane receptors. They are produced by enzymes in axon terminals (in response to Ca2+ entry), and simply diffuse from their sites of origin in one cell into the intracellular fluid of other neurons or effector cells, where they bind to and activate proteins. • Examples: • Nitric oxide (NO) is produced by nitric oxide synthetase (eNOS, nNOS, iNOS) and undergoes very rapid degradation. Once in the target cell, it activates cGMP signaling pathways. • Carbon monoxide and hydrogen sulfide are also emitted by neurons as signals. 83 Purine Neurotransmitters • Other nontraditional neurotransmitters include the purines, ATP and adenosine, which act principally as neuromodulators. • ATP is present in all pre-synaptic vesicles and is co-released with one or more of the classical neurotransmitters in response to Ca2+ influx into the terminal. • Adenosine is derived from ATP via extracellular enzymatic activity. • Both presynaptic and postsynaptic receptors have been described for adenosine, and the roles these substances play in the nervous system and other tissues is an active area of research. 84 Neuroeffector Communication • Many neurons synapse not on other neurons but on muscle and gland cells. • The events that occur at neuroeffector junctions are similar to those at synapses between neurons. • The neurotransmitter is released from the efferent neuron, diffuses to the surface of the effector cell, where it binds to receptors on that cell’s plasma membrane. • The receptors on the effector cell may be either ionotropic or metabotropic. 85 Structure of the Nervous System Fig. 6-37 86 Central Nervous System: Brain Fig. 6-38 87 Forebrain • The cerebrum consists of the right and left cerebral hemispheres as well as certain other structures on the underside of the brain. The central core of the forebrain is formed by the diencephalon. • The cerebral hemispheres consist of the cerebral cortex, an outer shell of gray matter composed primarily of cell bodies that give the area a gray appearance, and an inner layer of white matter, composed primarily of myelinated fiber tracts. • This overlies cell clusters, which are also gray matter and are collectively termed the subcortical nuclei. The fiber tracts consist of the many nerve fibers that bring information into the cerebrum, carry information out, and connect different areas within a hemisphere. 88 Forebrain: Cerebral Cortex • The cortex layers of the left and right cerebral hemispheres, although largely separated by a deep longitudinal division, are connected by a massive bundle of nerve fibers known as the corpus callosum. • The cortex of each cerebral hemisphere is divided into four lobes: – – – – Frontal Parietal Occipital Temporal. • The cortex is 3 mm in thickness but is highly folded. This results in an area containing cortical neurons that is four times larger than it would be if unfolded, without appreciably increasing the volume of the brain. 89 Forebrain: Cerebral Cortex • This folding results in the characteristic external appearance of the human cerebrum, with its sinuous ridges called gyri (singular, gyrus) separated by grooves called sulci (singular, sulcus). • The cells of the human cerebral cortex are organized in six distinct layers, composed of two basic types: pyramidal cells (named for the shape of their cell bodies) and nonpyramidal cells. • The pyramidal cells form the major output cells of the cortex, sending their axons to other parts of the cortex and to other parts of the central nervous system. • Nonpyramidal cells are mostly involved in receiving inputs into the cortex and in local processing of information. 90 Forebrain: Cerebral Cortex • The cerebral cortex is the integrating area of the nervous system. • In the cerebral cortex, basic afferent information is collected and processed into meaningful perceptual images, and control over the systems that govern the movement of the skeletal muscles is refined. • The subcortical nuclei are heterogeneous groups of gray matter that lie deep within the cerebral hemispheres. Predominant among them are the basal nuclei (also known as basal ganglia), which play an important role in controlling movement and posture and in more complex aspects of behavior. 91 Forebrain: Diencephalon • The diencephalon, which is divided in two by the narrow third cerebral ventricle, contains the thalamus, hypothalamus, and epithalamus. • The thalamus is a collection of several large nuclei that serve as synaptic relay stations and important integrating centers for most inputs to the cortex, and plays a key role general arousal. • The thalamus also is involved in focusing attention. For example, it is responsible for filtering out extraneous sensory information. 92 Forebrain: Diencephalon • The hypothalamus lies below the thalamus and is on the undersurface of the brain and it contains different cell groups and pathways that form the master command center for neural and endocrine coordination. • Behaviors having to do with preservation of the individual (for example, eating and drinking) and preservation of the species (reproduction) are among the many functions of the hypothalamus. • The hypothalamus lies directly above and is connected by a stalk to pituitary gland, an important endocrine structure that the hypothalamus regulates (Chapter 11). 93 Forebrain: Diencephalon • The epithalamus is a small mass of tissue that includes the pineal gland, which has a role in regulating biological rhythms. • Some of the forebrain areas, consisting of both gray and white matter, are also classified together in a functional system called the limbic system. • Structures within the limbic system are associated with learning, emotional experience and behavior, and a wide variety of visceral and endocrine functions (see Chapter 8). 94 Limbic System Fig. 6-40 95 Cerebellum • Although the cerebellum does not initiate voluntary movements, it is an important center for coordinating movements and for controlling posture and balance. • To carry out these functions, the cerebellum receives information from the muscles and joints, skin, eyes and ears, viscera, and the parts of the brain involved in control of movement. • Although the cerebellum’s function is almost exclusively motor, it is implicated in some forms of learning. 96 Brainstem • All the nerve fibers that relay signals between the forebrain, cerebellum, and spinal cord pass through the brainstem. • Running through the core of the brainstem and consisting of loosely arranged neuron cell bodies intermingled with bundles of axons, is the reticular formation, the one part of the brain absolutely essential for life. • It receives and integrates input from all regions of the central nervous system and processes a great deal of neural information. The reticular formation is involved in motor functions, cardiovascular and respiratory control, and the mechanisms that regulate sleep and wakefulness and focus of attention. 97 Brainstem • Some reticular formation neurons are clustered together, forming brainstem nuclei and integrating centers. • These include the cardiovascular, respiratory, swallowing, and vomiting centers, all of which we will discuss in later chapters. The reticular formation also has nuclei important in eyemovement control and the reflex orientation of the body in space. • The brainstem contains nuclei involved in processing information for 10 of the 12 pairs of cranial nerves. These are the peripheral nerves that connect directly with the brain and innervate the muscles, glands, and sensory receptors of the head, as well as many organs in the thoracic and abdominal cavities. 98 Cranial Nerves 99 Spinal Cord • The spinal cord lies within the bony vertebral column and is a slender cylinder of soft tissue about as big around as the little finger. • The central butterfly-shaped area of gray matter is composed of interneurons, the cell bodies and dendrites of efferent neurons, the entering axons of afferent neurons, and glial cells. • The regions of gray matter projecting toward the back of the body are called the dorsal horns, whereas those oriented toward the front are the ventral horns. • The gray matter is surrounded by white matter, which consists of groups of myelinated axons. 100 Spinal Cord • These groups of fiber tracts run longitudinally through the cord, some descending to relay information from the brain to the spinal cord, others ascending to transmit information to the brain. Pathways also transmit information between different levels of the spinal cord. • Groups of afferent fibers that enter the spinal cord from the peripheral nerves enter on the dorsal side of the cord via the dorsal roots. Small bumps on the dorsal roots, the dorsal root ganglia, contain the cell bodies of these afferent neurons. • The axons of efferent neurons leave the spinal cord on the ventral side via the ventral roots. A short distance from the cord, the dorsal and ventral roots from the same level combine to form a spinal nerve, one on each side of the spinal cord. 101 Central Nervous System: Spinal Cord Fig. 6-41 102 Peripheral Nervous System • Neurons in the peripheral nervous system transmit signals between the central nervous system and receptors and effectors in all other parts of the body. • The peripheral nervous system has 43 pairs of nerves: 12 pairs of cranial nerves and 31 pairs that connect with the spinal cord as the spinal nerves. • The 31 pairs of spinal nerves are designated by the vertebral levels from which they exit: cervical (8), thoracic (12), lumbar (5), sacral (5), and coccygeal (1). 103 Peripheral Nervous System • The eight pairs of cervical nerves control the muscles and glands and receive sensory input from the neck, shoulders, arms, and hands. • The 12 pairs of thoracic nerves are associated with the chest and upper abdomen. • The five pairs of lumbar nerves are associated with the lower abdomen, hips, and legs. • The five pairs of sacral nerves are associated with the genitals and lower digestive tract. (A single pair of coccygeal nerves associated with the tailbone brings the total to 31 pairs.) 104 Peripheral Nervous System • These peripheral nerves can contain nerve fibers that are the axons of efferent neurons, afferent neurons, or both. • All the spinal nerves contain both afferent and efferent fibers, whereas some of the cranial nerves contain only afferent fibers or only efferent fibers. • Efferent neurons carry signals out from the central nervous system to muscles or glands. The efferent division of the peripheral nervous system is more complicated than the afferent, being subdivided into a somatic nervous system and an autonomic nervous system. 105 Spinal Nerves Fig. 6-42 106 107 Autonomic Nervous System • The gastrointestinal tract has the enteric nervous system, and although often classified as a subdivision of the autonomic efferent nervous system, it also includes sensory neurons and interneurons. • Anatomical and physiological differences within the autonomic nervous system are the basis for its further subdivision into sympathetic and parasympathetic divisions. • The neurons of the sympathetic and parasympathetic divisions leave the central nervous system at different levels—the sympathetic fibers from the thoracic (chest) and lumbar regions of the spinal cord, and the parasympathetic fibers from the brainstem and the sacral portion of the spinal cord. 108 Autonomic Nervous System • Sympathetic division is also called the thoracolumbar division, has short pre-ganglionic and long post-ganglionic synapses. The major neurotransmitters are ACh at the pre-ganglionic synapse and usually NE and Epi at the post-ganglionic synapse. This is the “Flight or Fight” response system. • Parasympathetic is called the craniosacral division, it has long pre-ganglionic and short post-ganglionic synapses. The major neurotransmitter is ACh at both pre- and post-ganglionic synapses. This is the “Rest and Digest” system. 109 Autonomic Nervous System • In addition to the classical autonomic neurotransmitters just described, there is a widespread network of postganglionic neurons recognized as nonadrenergic and noncholinergic. • These neurons use nitric oxide and other neurotransmitters to mediate some forms of blood vessel dilation and to regulate various gastrointestinal, respiratory, urinary, and reproductive functions. • The great majority of acetylcholine receptors in the autonomic ganglia are nicotinic receptors. In contrast, the acetylcholine receptors on smooth muscle, cardiac muscle, and gland cells are muscarinic receptors. 110 Autonomic Nervous System • The cholinergic receptors on skeletal muscle fibers, innervated by the somatic motor neurons, not autonomic neurons, are nicotinic receptors. • One set of postganglionic neurons in the sympathetic division never develops axons. Instead, they form the adrenal medulla. • Upon activation by preganglionic sympathetic axons, cells of the adrenal medulla release a mixture of about 80 percent epinephrine and 20 percent norepinephrine into the blood (plus small amounts of other substances, including dopamine, ATP, and neuropeptides). 111 Autonomic Nervous System • These catecholamines, properly called hormones rather than neurotransmitters in this circumstance, are transported via the blood to effector cells having receptors sensitive to them. • The heart and many glands and smooth muscles are innervated by both sympathetic and parasympathetic fibers; that is, they receive dual innervation. • Whatever effect one division has on the effector cells, the other division usually has the opposite effect. • Moreover, the two divisions are usually activated reciprocally; that is, as the activity of one division increases, the activity of the other decreases. 112 Autonomic nervous system Fig. 6-44 113 PSNS vs SNS Fig. 6-46 114 Cerebrospinal Fluid Fig. 6-47 115 Physical Support of the CNS • Bone serves to support and to protect the structures of the CNS and PNS. • Cranium • Vertebrae • Meninges are the membranes that line the structures and add additional support and protection. • Dura mater • Arachnoid mater • Pia mater • Cerebrospinal fluid (CSF) protects and cushions the structures. 116 The Meninges • There are three layers of the meninges. From external to internal they are: dura mater, arachnoid mater, and pia mater. • The job of the meninges is to: 1. Cover and protect the CNS 2. Protect blood vessels and enclose the venous sinuses 3. Contain cerebrospinal fluid 4. Form partitions in the skull 117 The Meninges • The subarachnoid space is filled with CSF and contains the largest blood vessels serving the brain. In the superior sagittal sinus the arachnoid villi absorb the CSF into the venous blood system. • The pia mater clings to the brain and contains a network of blood vessels. • Meningitis is an inflammation of the meninges and is a serious threat to the brain since bacterial or viral meningitis can spread to the CNS. If the brain itself is inflamed it is called encephalitis. Meningitis is usually diagnosed by examining the CSF obtained via a lumbar puncture for microbes or viruses. 118 Cerebrospinal Fluid (CSF) • CSF is the extracellular fluid of the CNS and it is secreted by ependymal cells of the choroid plexus. It circulates through the subarachnoid space and ventricles and is reabsorbed by arachnoid villi. • Total volume of CSF present at any given time is approximately 125–150 mL. The choroid plexus produces 400–500 mL/day, so the entire contents are recycled three times a day. • This is important to maintaining a stable and optimal environment. 119 Hydrocephalus • Hydocephalus is “water on the brain”. • It is an accumulation of CSF in the brain that is often caused by tumors. • In newborns it results in enlargement of the head. In adults (whose skull bones have fused) it puts pressure on the brain and causes brain damage. It is treated by inserting a shunt to drain the fluid. 120 Blood-Brain Barrier • This is a protective mechanism that helps maintain a stable environment for the brain. • Substances in the brain’s capillaries are separated from the extracellular space by the continuous endothelium of the capillary walls and a thick basal lamina surrounding the capillaries. The “feet” of the astrocytes surrounding the capillaries also contribute. • These capillaries are the least permeable ones in the body. This barrier is very selective. Things that are highly lipid-soluble cross easily. 121 Cerebrovascular Accidents • Otherwise known as a stroke, CAs can be caused by a decreased blood supply or a hemorrhage. • An ischemic stroke is one caused by the occlusion of cerebral arteries, usually by a blood clot that blocks an artery or by the rupture of an atherosclerotic plaque . If it is detected early enough we can give a “clot busting” drug called TPA (tissue plasminogen activator). This dissolves the clot and restores blood flow to the area. • A hemorragic stroke is one where the blood vessel has ruptured. The best treatment that we have available is to try to cauterize the vessel (if we can get to it) and to alleviate the pressure on the brain caused by the bleeding. 122 Head Injuries • Head injuries are the leading cause of accidental death in North America. • Hard blows to the head result in a coup injury (the site of the blow) as well as a contrecoup (where the brain hits the other side of the skull). • A concussion is an alteration of brain function following a blow to the head. This is usually temporary and the victim can show signs of dizziness, or may lose consciousness. Multiple concussions can cause cumulative damage (boxers and football player have to be carefully monitored for this). • A contusion is bruising of the brain. This always causes some permanent damage. If it occurs in the brainstem it can cause coma or death. • Head blows may also result in subdural or subarachnoid hemorrhages which can cause permanent neurological damage and death. • Cerebral edema can also be caused by a blow to the head. This can cause permanent damage or death by putting pressure on the brain. 123