Glue Ball - topsofscv.org

Glue Ball Chemistry
Making Rubber from Glue
Glue Ball Chemistry - 1
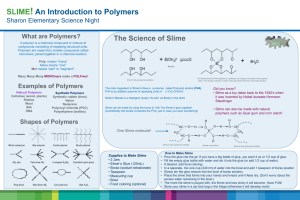
What is a Polymer?
Polymers are very large molecules made of a chain of many smaller molecules bonded together.
The prefix “poly” means many.
The small molecules that are used to create polymers are called monomers .
The prefix
“mono” means one.
Most polymers are made of organic compounds that contain the elements carbon and hydrogen, but may also include other elements like oxygen and nitrogen.
Glue Ball Chemistry - 2
How are Polymers made?
Polymers form when chemical bonds link large numbers of monomers together in a repeating pattern.
Think of polymers like chains of many paper clips connected together. This is why chemists often call them “polymer chains.” Each paper clip would represent a monomer molecule.
When two different monomers combine, they form a copolymer
Glue Ball Chemistry - 3
What is Elmer’s Glue?
Elmer’s glue is a mixture of a polymer called polyvinyl acetate and water.
The long “chains” of the polymer can slide past each resulting in a viscous liquid.
Below is a picture of the chemical structure of a small section of the polyvinyl acetate chain:
Glue Ball Chemistry - 4
How does the glue become rubber?
Step 1
When Borax, also known as Sodium Perborate
(Na2B4O7), dissolves in water, borate ions are formed in 2 successive reactions:
Glue Ball Chemistry - 5
How does the glue become rubber?
Step 2
The borate ions react with the polyvinyl acetate to form polyvinyl alcohol.
Glue Ball Chemistry - 6
How does the glue become rubber?
Step 3
The borate ions combine with the polyvinyl alcohol chains to link them together in cross-links.
Glue Ball Chemistry - 7
Why does the glue become rubber?
Glue Ball Chemistry - 8
The Experiment
1. Make a saturated solution of Borax in water.
2. Measure 5 ml of water in a cup, place a mark on the cup at the 5 ml level, and discard the water.
3. Pour Elmer’s white glue into the cup until it reaches the 5 ml mark you made in step 1.
4. Pour 20 ml of the Borax solution into the cup containing the glue-water mixture and stir.
5. After the material becomes like rubber, scrape it out and form it into a ball, squeezing out the excess liquid into a wastebasket.
The liquid glue has been transformed into a rubber ball by polymer cross-linking.
Glue Ball Chemistry - 9