Reduction of GHG Emissions _ Urea Recovery _ Nov 29 2013
advertisement

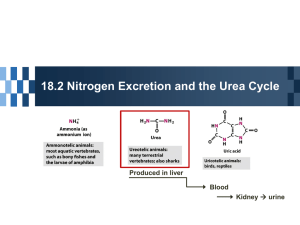
STRATEGIES FOR REDUCING GREENHOUSE GAS EMISSIONS FROM LIVESTOCK WASTES Steven A. Nyanzi Department of Chemistry Makerere University 29th November 2013 Seminar Room, Department of Chemistry, College of Natural Sciences, Makerere University OVERVIEW • • • • • • • • • • Background Livestock and GHG Emissions Approaches for reduction of NH3 Emissions Emission reduction via urea recovery? Harvesting & stabilizing urea in urine Effect of Temp and pH on urea in urine Pre-concentration with an urease inhibitor Extraction of urea as clathrate Characterization Conclusion Background on Climate Change • Over 180 Countries are signatory to United Nations Framework Convention on Climate Change (UNFCC) by early 1990s • UNFCC aims at stabilizing the conc’n of GHG in the atmosphere and reduce –ve impacts of climate change • Green House Gases (GHG) include: CO2, CH4, N2O, • Global Warming Potential (GWP): I kg CH4= 23 times CO2; I kg N2O = 310 times CO2 IPCC on NITROUS OXIDE • IPCC Guidelines for estimating N2O emissions from agricultural systems 1) Direct emissions of N2O from agric fields 2) Direct emissions of N2O in animal production systems 3) Indirect emissions of N2O derived N used in agriculture • IPCC Guidelines for National Greenhouse Gas Inventories (IPCC, 1997) excludes 2 and 3 ! Estimates of nitrous oxide emissions from agricultural systems worldwide, directly from agricultural fields (direct) from animal waste management systems (AWMS) and from indirect sources (indirect). Livestock and GHG Emissions • Uganda has one of the fastest growing population growth rate (3.3% per annum) • Livestock = cattle, goats, sheep, pigs, chicken • Cattle pop. in Uganda is about 11.4 million • Cattle belching and fatting produce about 100 – 200 L of CH4 per cow per day • 90% of urea + other urea derivatives ((i.e., allantoin, uric acid) are excreted in animal urine • Urea, Allantoin and Uric acid break down to produce CH4, CO2 and N2O Mechanism for Conversion of Allantoin to Urea -O H N O O O C CH Allantoinase C NH C -O CH H2N N H NH2 N H NH2 Allantoate Allantoin O O O O C H2N O O C N H O N H H2N Állantoate Aminohydrolase CH N H NH2 Urea NH2 O Allantoate H N C -O CH OH Ureidoglycolate NH2 C O Conversion of Allantoin to Urea O H N H N HN 1) Hydration O O N H N H H N O CH C N H NH NH2 3) Decarboxylation Allantoin Uric acid O C C 2) Oxidation O O O NHCNH2 H O O C C H2N OH + O 2 HN Glyoxalic acid O Allantoin H2N Urea Mechanism Contd. O C -O O H N CH NH2 C Ureidoglycolate Urea-Lyase OH H2N NH2 O Urea Ureidoglycolate O O HC O - Gloxylate Processes Contd. O H N C -O CH NH2 C Ureidoglycolate Aminohydrolase OH O + CO2 O Ureidoglycolate 2 NH3 O HC O - Gloxylate N2O NH3 Nitrification/Denitrification 2 NH3 + O2 Oxidation N2O + 3 H2O APPROACHES FOR REDUCTION OF NH3 EMISSIONS • A) Land application of animal manure • B) Dietary manipulation of crude protein • C) Use of animal feed diets containing tannins and polyphenols • D) Use of additives to livestock wastes – a variety of additives including urease inhibitors Emissions Reduction via Urea Recovery ? • Urea 16th most highly produced substance • World production ~ 2.0 x108 tonnes/ year • Applications : Fertilizer (> 90%), Liu et al (2003) Resins (melanine-formaldehyde, etc) Supplementary protein source Pharmaceutical, fermenting & brewing, petroleum industry, soap production Hydrogen carrier (Renewable Energy) ! ! Harvesting & Stabilizing Urea in Urine • • • • • • • • Challenges in urine collection Enzymatic degradation of urea Production and control of stench Local materials as urease inhibitors Indigenous practices Environmental benefits –Less NH3 emissions NH3 emissions responsible FPM (d<2.5 μm) Improved Agricultural productivity - Fertilizers Determination of Urea in Urine • Colorimetric methods most widely used • Indirect Methods: Determine products of urea e.g., NH3 Berthelot reaction (λmax 640 nm) Macro-method but NH3 interferes • Direct Methods : Determine urea directly i.e., Fearon’s method (λ max 525 nm) Modified Fearon’s method (Nyanzi et al, 2010) Effect of pH on urea concentration pH 3 Urea concentration (g/dL) 1.2 pH 4 1 pH 5 0.8 pH 6 0.6 pH 7 0.4 pH 8 pH 9 0.2 pH 10 0 1 3 5 7 Time (days) 9 11 13 pH 11 pH 12 Pre-concentration • • • • • • • Filter to remove solids Stabilization of urine extract at pH 9 Pass through bleaching material Heating extract at 96 OC for max 20 min Heating at < 96 OC for longer Conversion of allantoin to urea Use of a solar drier to reduce moisture Urine pH against Time in Days 14 D 13 C 12 11 pH 10 9 A 8 B 7 0 2 4 6 8 10 Time (Days) A and B without substrate X; C and D with substrate X 12 Urea content in Urine against Time in Days 0.7 C 0.6 D 0.5 Abs 0.4 0.3 0.2 B A 0.1 0 2 4 6 8 10 12 Time (Days) A and B without substrate X; C and D with substrate X 14 Urea-Hydrocarbon Clathrates • Previous Applications: – Purify fatty acids from fats and vegetables – Separate straight chain from branched chain or cyclic compounds – Separate different petroleum fuel fractions • Current Application – Use urea to extract FAs from plant oils ? – Use these FAs to extract urea from urine Extraction of Urea as Clathrate Moles of Hydrocarbon Heptane (n-C7H16) Molar proportion of urea in Complex 6 Decane (n-C10H22) 8.3 Hexadecane (n-C16H34) 12 Octacosane (n-C28H58) 21 n-Alkane-Urea complex: (a) cross section; (b) Hydrogen bonding (Courtesy of A. E. Smith) Infrared spectra of: (a) extracted urea (b) pure urea Urea from indigenous and Friesian cattle urine Conclusion • Reduction of GHG Emissions from livestock wastes is possible • Urea stabilization, enrichment and extraction have lots of potential in odour control and urea production from livestock wastes! 3.08x103 tonnes urea could be recovered everyday! • Production of urea from livestock wastes can create wealth, protect the environment & reduce ammonia emissions • Sustainability through the process (Fertilizer Animal feeds- Animal wastes-Urea) A cow excreting liquid gold Acknowledgement • • • • • • • • Organizers of this Interface Mr. G. Wamala Mr. Lubwama Mr. Magada Dr. J. Hawumba Dr. Emmanuel Tebandeke Mr. Hassan Kigozi Wasswa Mr. Christopher Biteinensha (FTIR spectra) I THANK YOU