States of Matter

Section 3.1
States of Matter
Kinetic Theory of Matter
Why do materials behave the way they do?
Kinetic Theory of Matter
• A theory that all matter is made up of tiny particles (atoms or molecules, depending on the substance) that are in CONSTANT
MOTION
• As temperature increases, the particles move faster
• At the same temperature, heavier particles move more slowly than lighter particles
Kinetic Theory of Matter
• The motion of the individual particles (i.e. energy) and the forces of attraction between them determines the state of matter
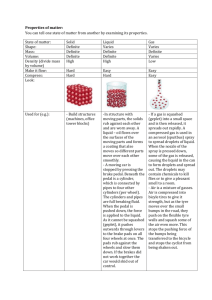
States of Matter
• Solid
• Liquid
• Gas
• Plasma
• Bose Einstein
Condensate (BEC)
Behavior of Gases
• Particles of a gas are in constant, random motion at very high speeds
• Gases have more energy than liquids or solids of the same material
Behavior of Gases
• The motion of one particle is not affected by the other particles
• The attraction between individual particles is so small that it can be ignored
• The constant motion at high speeds allows the gas to fill its container, even if another gas is in the space
Behavior of Liquids
• Particles of a liquid move more slowly than gases and are more closely packed
• Liquids have less energy than gases, but more than solids
Behavior of Liquids
• Attractive forces between the particles is strong enough to affect the motion of other particles, to a certain degree
• Particles can still move, but not as quickly as gases
• Mixing occurs more slowly
Behavior of Liquids
• No Definite Shape
– Forces of attraction are not strong enough to hold particles in one place, so they are free to move past one another and fill the shape of their container
• Definite Volume
– The attractive forces are strong enough to keep individual particles close, preventing them from breaking away from the group
Behavior of Solids
• Particles of a solid will vibrate in place, but will not move away from a fixed location
• Solids have the least amount of energy of all states of matter
Behavior of Solids
• The particles of a solid are attracted to each other very strongly
– They move much less than liquids or gases
• This results in a definite shape and definite volume