Principles of BIOCHEMISTRY
advertisement
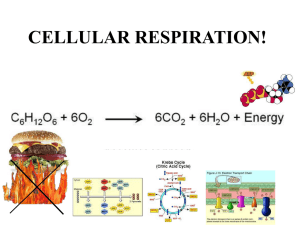
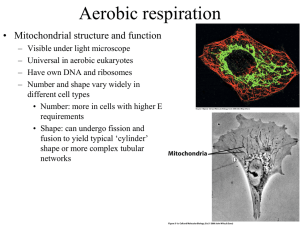
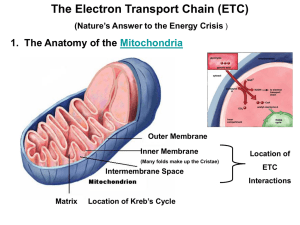
Chapter 14 - Electron Transport and Oxidative Phosphorylation • The cheetah, whose capacity for aerobic metabolism makes it one of the fastest animals Oxidative Phosphorylation in Mitochondria • Oxidative phosphorylation is the process by which NADH and FADH2 are oxidized and ATP is formed • NADH and FADH2 are reduced coenzymes from the oxidation of glucose by glycolysis and the citric acid cycle The Respiratory Electron-transport Chain (ETC) is a series of enzyme complexes embedded in the inner mitochondrial membrane, which oxidize NADH and QH2. Oxidation energy is used to transport protons across the inner mitochondrial membrane, creating a proton gradient ATP synthase is an enzyme that uses the proton gradient energy to produce ATP Mitochondria are energy centers of a cell Cytosol Mitochondria Fig 14.2 Fig 14.6 Structure of the mitochondrion • Final stages of aerobic oxidation of biomolecules in eukaryotes occur in the mitochondrion • Site of citric acid cycle and fatty acid oxidation which generate reduced coenzymes • Contains electron transport chain to oxidize reduced coenzymes Overview of oxidative phosphorylation Fig 14.1 Electron Flow in Oxidative Phosphorylation • Five oligomeric assemblies of proteins associated with oxidative phosphorylation are found in the inner mitochondrial membrane • Complexes I-IV contain multiple cofactors, and are involved in electron transport • Electrons flow through complexes I-IV • Complexes I, III and IV pump protons across the inner mitochondrial membrane as electrons are transferred • Mobile coenzymes: ubiquinone (Q) and cytochrome c serve as links between electron transport complexes • Complex IV reduces O2 to water • Complex V is ATP synthase, which uses the generated proton gradient across the membrane to make ATP Cofactors in Electron Transport • NADH donates electrons two at a time to complex I of the electron transport chain • Flavin coenzymes are then reduced (Complex I) FMN FMNH2 (Complex II) FAD FADH2 • FMNH2 and FADH2 donate one electron at a time to ubiquinone (U or coenzyme Q) • All subsequent steps in electron transport proceed by one electron transfers Mobile electron carriers 1. Ubiquinone (Q) Q is a lipid soluble molecule that diffuses within the lipid bilayer, accepting electrons from Complex I and Complex II and passing them to Complex III. 2. Cytochrome c Associated with the outer face of the inner mitochondrial membrane. Transports electrons from Complex III to Complex IV. Iron in metalloenzymes • Iron undergoes reversible oxidation and reduction: Fe3+ + e- (reduced substrate) Fe2+ + (oxidized substrate) • Enzyme heme groups and cytochromes contain iron • Nonheme iron exists in iron-sulfur clusters (iron is bound by sulfide ions and S- groups from cysteines) • Iron-sulfur clusters can accept only one e- in a reaction Iron-sulfur clusters • Iron atoms are complexed with an equal number of sulfide ions (S2-) and with thiolate groups of Cys side chains • Heme consists of a tetrapyrrole Porphyrin ring system complexed with iron Heme Fe(II)-protoporphyrin IX Complex I. NADH-ubiquinone oxidoreductase • Transfers two electrons from NADH as a hydride ion (H:-) to flavin mononucleotide (FMN), to iron-sulfur complexes, to ubiquinone (Q), making QH2 • About 4 protons (H+) are translocated across the inner mitochondrial membrane per 2 electrons transferred Fig 14.9 Complex II. Succinate-ubiquinone oxidoreductase • Also known as succinate dehydrogenase complex • Transfers electrons from succinate to flavin adenine dinucleotide (FAD) as a hydride ion (H:-), to an iron-sulfur complex, to ubiquinone (Q), making QH2 • Complex II does not pump protons Fig 14.11 Complex III. Ubiquinol-cytochrome c oxidoreductase • Transfers electrons from QH2 to cytochrome c, mediated by ironsulfur and other cytochromes • Electron transfer from QH2 is accompanied by the translocation of 4 H+ across the inner mitochondrial membrane Fig 14.14 Complex IV. Cytochrome c oxidase • Uses four-electrons from the soluble electron carrier cytochrome c to reduce oxygen (O2) to water (H2O) • Uses Iron atoms (hemes of cytochrome a) and copper atoms • Pumps two protons (H+) across the inner mitochondrial membrane per pair of electrons, or four H+ for each O2 reduced O2 + 4 e- + 4H+ Fig 14.19 2 H2O Complex V: ATP Synthase • F0F1 ATP Synthase uses the proton gradient energy for the synthesis of ATP • Composed of a “knob-and-stalk” structure • F1 (knob) contains the catalytic subunits • F0 (stalk) has a proton channel which spans the membrane. • Estimated passage of 3 protons (H+) per ATP synthesized Knob-and-stalk structure of ATP synthase Prentice Hall c2002 Chapter 14 19 Mechanism of ATP Synthase • There are 3 active sites, one in each b subunit • Passage of protons through the Fo channel causes the c-e-g unit to rotate inside the a3b3 hexamer, opening and closing the bsubunits, which make ATP Prentice Hall c2002 Chapter 14 20 Fig 14.20 Transport of ATP, ADP and Pi across the inner mitochondrial membrane • Adenine nucleotide translocase: unidirectional exchange of ATP for ADP (antiport) • Symport of Pi and H+ is electroneutral The P:O Ratio molecules of ADP phosphorylated P:O ratio = ----------------------------------------atoms of oxygen reduced • Translocation of 3H+ required by ATP synthase for each ATP produced • 1 H+ needed for transport of Pi, ADP and ATP • Net: 4 H+ transported for each ATP synthesized Calculation of the P:O ratio Complex I III IV #H+ translocated/2e4 4 2 Since 4 H+ are required for each ATP synthesized: For NADH: 10 H+ translocated / O (2e-) P/O = (10 H+/ 4 H+) = 2.5 ATP/O For succinate (FADH2) substrate = 6 H+/ O (2e-) P/O = (6 H+/ 4 H+) = 1.5 ATP/O Regulation of Oxidative Phosphorylation • Overall rate of oxidative phosphorylation depends upon substrate availability and cellular energy demand • Important substrates: NADH, O2, ADP • In eukaryotes intramitochondrial ratio ATP/ADP is a secondary control mechanism • High ratio inhibits oxidative phosphorylation as ATP binds to a subunit of Complex IV