6. Alkenes: Structure and Reactivity
advertisement

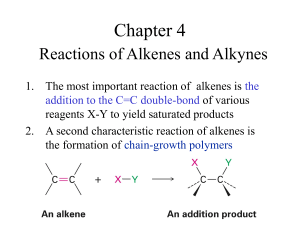
Chapter 7 Alkenes: Structure and Reactivity Chapter 7 Alkenes: Structure and Reactivity 7.2 Calculating Degree of Unsaturation Relates molecular formula to possible structures Degree of unsaturation: number of multiple bonds or rings Formula for a saturated acyclic compound is CnH2n+2 Alkene has fewer hydrogens than an alkane with the same number of carbons —CnH2n because of double bond Each ring or multiple bond replaces 2 H's Example: C6H10 Saturated is C6H14 therefore 4 H's are not present This has two degrees of unsaturation Two double bonds? or triple bond? or two rings? or ring and double bond? Degree of Unsaturation With Other Elements Organohalogens (X: F, Cl, Br, I) Halogen replaces hydrogen C4H6Br2 and C4H8 have one degree of unsaturation Degree of Unsaturation (Continued) Organoxygen compounds (C,H,O) – Oxygen forms 2 bonds these don't affect the formula of equivalent hydrocarbons May be ignored in calculating degrees of unsaturation Organonitrogen Compounds Nitrogen has three bonds So if it connects where H was, it adds a connection point Subtract one H for equivalent degree of unsaturation in hydrocarbon Summary - Degree of Unsaturation Count pairs of H's below CnH2n+2 Add number of halogens to number of H's (X equivalent to H) Ignore oxygens (oxygen links H) Subtract N's - they have two connections Cis-Trans Isomerism in Alkenes Rotation of bond is prohibitive This prevents rotation about a carbon-carbon double bond (unlike a carbon-carbon single bond). 7.6 Stability of Alkenes Cis alkenes are less stable than trans alkenes Less stable isomer is higher in energy Stability of Alkenes (Continued): Comparing Stabilities of Alkenes Evaluate heat given off when C=C is converted to C-C More stable alkene gives off less heat trans-Butene generates 5 kJ less heat than cis-butene 7.6 Stability of Alkenes Less stable isomer is higher in energy tetrasubstituted > trisubstituted > disubstituted > monosusbtituted Hydrogenation Data Helps to Determine Stability 7.7 Electrophilic Addition of Alkenes General reaction mechanism of electrophilic addition Attack on electrophile (such as HBr) by bond of alkene Produces carbocation and bromide ion Carbocation is an electrophile, reacting with nucleophilic bromide ion Electrophilic Addition of Alkenes (Continued): Electrophilic Addition Energy Path Two step process First transition state is high energy point First step is slower than second Electrophilic Addition of Alkenes (Continued) The reaction is successful with HCl and with HI as well as HBr HI is generated from KI and phosphoric acid 7.8 Orientation of Electrophilic Additions: Markovnikov’s Rule In an unsymmetrical alkene, HX reagents can add in two different ways, but one way may be preferred over the other If one orientation predominates, the reaction is regioselective Markovnikov observed in the 19th century that in the addition of HX to alkene, the H attaches to the carbon with more H’s and X attaches to the other end (to the one with more alkyl substituents) This is Markovnikov’s rule Example of Markovnikov’s Rule Addition of HCl to 2-methylpropene Regiospecific – one product forms where two are possible If both ends have similar substitution, then not regiospecific Markovnikov’s Rule (restated) More highly substituted carbocation forms as intermediate rather than less highly substituted one Tertiary cations and associated transition states are more stable than primary cations Markovnikov’s Rule (restated) Definitions Regioisomers – two constitutional isomers that could result from an addition reaction. Regioselective – both regioisomers are formed, but one is formed in preference. “Regiospecific” – only one regiosisomer forms at the expense of the other. 7.9 Carbocation Structure and Stability Carbocations are planar and the tricoordinate carbon is surrounded by only 6 electrons in sp2 orbitals the fourth orbital on carbon is a vacant p-orbital the stability of the carbocation (measured by energy needed to form it from R-X) is increased by the presence of alkyl substituents Carbocation Structure and Stability (Continued) A plot of DH dissociation shows that more highly substitued alkyl halides dissociate more easily than less highly substituted ones Carbocation Structure and Stability (Continued) A inductive stabilized cation species Competing Reactions and the Hammond Postulate Normal Expectation: Faster reaction gives more stable intermediate Intermediate resembles transition state 7.11 Evidence for the Mechanism of Electrophilic Addition: Carbocation Rearrangments Carbocations undergo structural rearrangements following set patterns 1,2-H and 1,2alkyl shifts occur Goes to give most stable carbocation 2.4 Resonance Some molecules are have structures that cannot be shown with a single representation In these cases we draw structures that contribute to the final structure but which differ in the position of the bond(s) or lone pair(s) Such a structure is delocalized and is represented by resonance forms The resonance forms are connected by a double-headed arrow Resonance Hybrids A structure with resonance forms does not alternate between the forms Instead, it is a hybrid of the resonance forms, so the structure is called a resonance hybrid For example, benzene (C6H6) has two resonance forms with alternating double and single bonds In the resonance hybrid, the actual structure, all its C-C bonds are equivalent, midway between double and single 2.5 Rules for Resonance Forms Individual resonance forms are imaginary - the real structure is a hybrid (only by knowing the contributors can you visualize the actual structure) Resonance forms differ only in the placement of their or nonbonding electrons Different resonance forms of a substance do not have to be equivalent Resonance forms must be valid Lewis structures: the octet rule generally applies The resonance hybrid is more stable than any individual resonance form would be Curved Arrows and Resonance Forms We can imagine that electrons move in pairs to convert from one resonance form to another A curved arrow shows that a pair of electrons moves from the atom or bond at the tail of the arrow to the atom or bond at the head of the arrow 2.6 Drawing Resonance Forms Any three-atom grouping with a p orbital on each atom has two resonance forms Different Atoms in Resonance Forms Sometimes resonance forms involve different atom types as well as locations The resulting resonance hybrid has properties associated with both types of contributors The types may contribute unequally The “enolate” derived from acetone is a good illustration, with delocalization between carbon and oxygen 2,4-Pentanedione The anion derived from 2,4-pentanedione Lone pair of electrons and a formal negative charge on the central carbon atom, next to a C=O bond on the left and on the right Three resonance structures result