Major product
advertisement
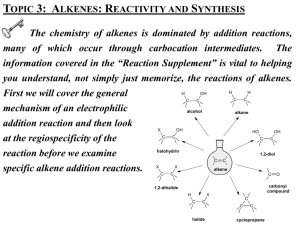
ALKENE AND ALKYNE REACTIONS Dr. Clower CHEM 2411 Spring 2014 McMurry (8th ed.) sections 7.7-7.8, 7.10-7.11, 10.3-10.4, 8.2-8.8, 8.10, 8.12, 9.3-9.8, 7.1, 8.1, 9.2, 9.9 Outline • Reactions of alkenes • Reactions of alkynes • Preparation of alkenes and alkynes • Synthesis • No reactions of alkyl halides (originally on syllabus) Reaction Charts • Help organize reaction details • Organize charts by reaction type, starting material, product • See webpage for template • Example: Reaction Type Oxymercurationdemercuration Starting Material Reagent Regiochemistry/ stereochemistry Rearrangement possible? Product Alkene 1. Hg(OAc)2, H2O 2. NaBH4 Markovnikov Anti addition no Alcohol Reactions of Alkenes I. II. III. IV. V. Allylic halogenation Electrophilic addition Reduction Oxidation Polymerization I. Allylic Halogenation • Similar to radical halogenation of alkanes • Alkene react with molecular halogen in the presence of heat or light • Alkyl halide is produced • Substitution of –X for –H at the allylic position • Most stable radical intermediate • Stabilized by resonance Allylic Halogenation • Another set of reagents: • N-bromosuccinimide (NBS), hn • Bromination only (no chlorination) • Product is a racemic mixture (if there is a stereocenter) Radical Stability • What is the major product of the reaction of 1-octene with NBS (in the presence of light)? • What is the major product of the reaction of 1-octene with NBS (in the presence of light)? • Reaction occurs at less sterically hindered carbon and produces the more stable C=C • What is the major product of the following reaction? II. Electrophilic Addition • Most common reaction of alkenes • Examples: • Break p bond of alkene • Form new s bonds to each C of double bond • Alkene is nucleophile; reacts with electrophile (HX, H2O, etc.) • Forms carbocation intermediate Electrophilic Addition • General mechanism: • Step 1: • Step 2: • Which step is RDS? Addition of Hydrogen Halides • HCl, HBr, HI • Example: 2-methylpropene + HBr • What is the major product of the following reaction? • Stereochemistry of product = racemic mixture • Carbocation intermediate is planar, sp2 hybridized • Regiochemistry of reaction • Which C gets the H? Which C gets the X? • Reaction is regiospecific for one product Regiochemistry of Electrophilic Addn. • Markovnikov’s Rule: • In the addition of HX (or H2O) to an alkene, the H will add to the carbon with the greater number of H’s already bonded to it • The X (or OH) attaches to the carbon with fewer H’s (the more substituted carbon) • Product = Markovnikov product • Opposite product = anti-Markovnikov or non-Markovnikov • Formed under specific conditions Markovnikov’s Rule Markovnikov’s Rule • Why is the Markovnikov product favored? • Look at reaction intermediate • Carbocation • Markovnikov addition forms the more stable R+ • 3º > 2º > 1º • More stable carbocation forms faster, will react to give product Markovnikov’s Rule • Draw and name the major product of the following reaction. • Draw and name the major product of the following reaction. • Expected product = • Actual product = • What happened? Carbocation Rearrangement • Carbocation intermediates can rearrange to form a more stable carbocation structure • Hydride shift = H:- moves from C adjacent to carbocation Carbocation Rearrangement • Alkyl groups can also shift • Typically methyl or phenyl (Major product) Anti-Markovnikov Addition of HBr • In the presence of peroxides • H2O2 or R2O2 • Free radical mechanism • Only HBr, not HCl or HI Addition of Halogens • X2 = Br2 or Cl2 (F2 too reactive, I2 unreactive) • Solvent = inert, nonaqueous • Stereochemistry = anti addition • Two X atoms add from opposite sides of the C=C • Product = a vicinal dihalide • Two X atoms on adjacent carbons Mechanism Addition of Halogens • Draw the major product of the following reaction. Addition of Halogens in the Presence of Water • Stereochemistry: X and OH add anti • Regiochemistry: X adds to the less substituted carbon OH adds to the more substituted carbon • Mechanism the same as addition of X2, except H2O is the nucleophile in the second step Mechanism Mechanism • Water attacks the carbon with the largest d+ • Results in OH on more substituted carbon • Draw the major product of the following reaction. Hydration • Addition of water • Three methods: A. Acid-catalyzed hydration B. Oxymercuration-demercuration C. Hydroboration-oxidation A. Acid-catalyzed hydration • Regiochemistry = Markovnikov • Acid catalyst typically H2SO4 or H3PO4 (or just H3O+) • Carbocation intermediate, so rearrangement can occur Mechanism • Draw the major product of the following reaction. B. Oxymercuration-demercuration • Step 1: Alkene reacts with mercuric acetate • Step 2: Reduction with sodium borohydride • Regiochemistry =Markovnikov • Stereochemistry = anti addition of OH and H • No rearrangements • Milder conditions than H3O+ • Electrophile is +HgOAc • Formed by dissociation of AcO-Hg-Oac • Intermediate is bridged mercurinium ion (similar to bromonium) Oxymercuration-demercuration • Draw the major product for each of the following reactions. C. Hydroboration-oxidation • Anti-Markovnikov product • Syn addition of H and OH (add on same side of C=C) • No rearrangements • THF stabilize highly reactive BH3 Hydroboration-oxidation • Mechanism of first step: • BH2 on the right because less steric hindrance • Leads to anti-Markovnikov product • Second step: H2O2/NaOH replace –BH2 with –OH • Keep same stereochemistry (syn) • Draw the major product of the following reaction. • Draw the major product formed when the following alkene undergoes (a) acid-catalyzed hydration, (b) oxymercurationdemercuration, and (c) hydroboration-oxidation. Oxidation and Reduction • What is oxidation? • What is reduction? • Classify these reactions as oxidation or reduction: • CH3─CH═CH2 → CH3─CH2─CH3 • CH3─CH2─OH → CH3─CO2H III. Reduction • Catalytic hydrogenation • Seen before with heat of hydrogenation (alkene stability) • Catalyst = metal, usually Pd, Pt, or Ni • Reaction takes place on metal surface • Stereochemistry = syn (both H’s add to same side of C=C) Mechanism Catalytic Hydrogenation • This reduction does not work with C=O, C=N, or benzene except at very high P or T, or with a special catalyst IV. Oxidation • Three types A. Epoxidation B. Hydroxylation C. Oxidative cleavage A. Epoxidation • Formation of epoxide • Cyclic ether • Example: • Reagent is peroxy acid (RCO3H) • Stereochemistry = syn • Another method: treat halohydrin with base: B. Hydroxylation • Formation of a 1,2-diol/glycol/vicinal diol • Methods: 1. Opening of epoxide using aqueous acid • Product is trans diol • Mechanism: Hydroxylation 2. Addition of osmium tetroxide (OsO4) or potassium permanganate (KMnO4) • How do you know these are both oxidizing agents? • Reaction includes some appropriate work-up • H2O2 or NaHSO3, H2O for OsO4 • HO- (aq) for KMnO4 • Stereochemistry = syn • Draw the major product of the following reaction. C. Oxidative Cleavage • Oxidize and alkene and split the C=C • Results in formation of 2 carbonyls • Type of carbonyls depends on alkene structure and the oxidizing agent used • Three types of oxidizing agents 1. Ozone 2. Potassium permanganate 3. Periodic acid Oxidative Cleavage 1. Ozone • Ozonolysis • Reagents: 1. O3 2. (CH3)2S or Zn, H3O+ • Products = 2 carbonyls (ketones or aldehydes) • Terminal alkenes give CO2 Oxidative Cleavage 2. KMnO4 • Reagents: KMnO4 (excess or concentrated) and heat or acid • Use heat and excess KMnO4 to split intermediate glycol • Products = 2 carbonyls (ketones or carboxylic acids) • Aldehydes oxidize to carboxylic acids in KMnO4 • Terminal alkenes still give CO2 Oxidative Cleavage 2. HIO4 • Specifically used to split glycol • Draw the major product for each of the following reactions. V. Polymerization • Polymer = large molecule synthesized by covalently linking single parts (monomers) • Biological polymers: proteins, cellulose, nucleic acids • Organic polymers: plastics • Chain-growth polymers: made from alkene monomers • Radical reaction Chain-growth Polymerization • Initiation by peroxides: • Propagation: • Termination: R─CH2CH2• + •CH2CH2─R → R─CH2CH2CH2CH2─R Chain-growth Polymers • Draw the structure of poly(vinyl chloride).