9-Stable isotopes in Oceanography
advertisement

δ13C of sediment organic matter in coastal Gulf of Mexico
Peterson,
1999
(original
data in
Sackett &
Thompson,
1963
Stable isotopes in Oceanography
Stable isotopes are used to:
• Trace the sources and sinks of material in the
environment
• Determine the extent and type of biogeochmical
processes which have acted on materials
• Provide information on paleooceanographic
conditions
• Experimentally trace specific elements using stable
isotope tracers i.e. 15N
by isotope ratio mass spectrometry
Light vs. heavy
isotopes
In all cases, the
light isotope is
the most
abundant i.e.
12C
>>
13C
16O
>>
18O
1H
32S
>> 2H
>>
34S
All these elements are analyzed as gases. So material for analyses must be
processed and converted to gas (usually by combustion)
Stable isotopes are STABLE
Total Mass is Conserved
Different isotopes of the elements are moved around the
Earth and partitioned slightly differently in different
compartments of the Earth system
Different isotopes
of the same
element have the
same chemistry
(same reactions,
same bonds, etc.),
but bond energies
differ however lighter isotopes have
higher vibrational
energy – therefore
more likely to react.
Slight differences
in reaction rates of
heavier vs. light
isotopes ultimately
results in
fractionation.
H3C COOH
H313C
COOH
Both are
acetic acid
Fundamentally, each isotopic form of a compound has
a slightly different free energy. Differences in free
energy result in different rate constants for the
different isotopic forms, and different equilibrium
constants. This results in slightly different partitioning
of the heavy and light isotopes in reactant and
product pools. (see Emerson an Hedges Chap 5 for
discussion)
Fig. 1.6. The extra neutron does make a very slight difference in some reactions; having an extra
neutron usually results in slower reactions. This reaction difference is fractionation. From Fry,
Stable Isotope Ecology, 2006
Isotopic composition of water - SMOW - Standard Mean
Ocean Water
This water is the reference material for isotopic analyses of D
(del-deuterium) and 18O.
Ocean water is made up of many different isotopic forms of
water, the main ones of which are:
H216O
Mass
18
H218O
20
DH16O,
19
D216O
20
DH18O
21
Water containing 18O instead of 16O is two mass units heavier
per molecule and thus is 12.5% more dense, and a tiny bit
slower to evaporate or react in a chemical reaction, resulting
in fractionation.
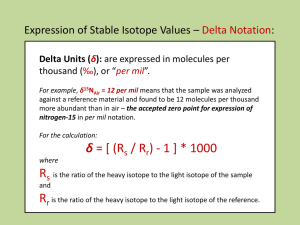
Definition of the del notation
using 13C as an example
13
13 C
C
12
12
C
C
sample
std
13
C
1000
13
C
12
C std
13 C
12
C sample
13C 13
1 1000
C
12 C std
R stands for the Ratio of the
Rsample
13
C
1
heavy to light isotope in
1000
Rstd
sample or standard
A positive value indicates the substance is enriched
in the heavy isotope (relative to the standard). Likewise, a
negative value of indicates the substance is depleted
in the heavy isotope (relative to the standard).
Example calculation for δ 13C value
Differences in abundance of isotopes is very small !
del 13C ={ [Rsample/Rstd]-1} *1000
# of atoms
13C-sample
10800
12C- sample
1000000
13C-std
11000
12C- std
1000000
A difference of only
200 atoms out of a
million gives a large
del 13C value!
[Rsample/Rstd]
Ratio 13C/12C sample =
0.0108
Ratio 13C/12C std =
0.011
Typical abundance of 13C
relative to 12C is 1.1%
0.98182
del 13C =
-18.18
Negative
value
indicates that
sample is
depleted in
13C relative to
standard
Some definitions
(note subtle differences in terminology:
Isotope Discrimination - The instantaneous difference in isotopic
composition, usually given in o/oo, between the parent substrate
undergoing reaction and the product, at any given instant in time.
Discrimination factor (after Fry, 2006):
D () = reactant - product
= [(Rsample/Rstd)-1] x 103
D is positive when light isotope reacts faster. Expressed in per mille
(mil) or o/oo.
Note: Emerson and Hedges use Difference Fractionation Factor:
ε = product - reactant
ε is negative when light isotope reacts faster.
Example for typical δ13C o/oo values: -22 - (-20) = -2
This reaction results in a -2
per mil shift to lighter
isotope. That is, the
product is isotopically
lighter by 2 o/oo
Hydrogen δ D
Carbon δ
13C
Oxygen δ
18O
Nitrogen δ
15N
Emerson & Hedges, Chap. 5
More definitions:
Fractionation factor () – (expressed in isotope ratios not del units)
The realized isotopic composition difference between reactants and
products.
= [13C/12C]products/ [13C/12C]reactants = Rproducts/Rreactants
For our example earlier the α was 0.98182
Value of will be close to 1 because isotopic differences are small!
The difference between Discrimination and Fractionation: A given
chemical reaction/process, say photosynthesis, may have associated
with it some isotope discrimination which would be constant if
conditions were constant and the substrate was unchanging. In the
real world scenario, conditions are variable and discrimination will
change over time, ultimately producing some net isotope
Fractionation.
Fractionation
factor for
carbon in
photosynthesis
is the same for
marine and
terrestrial
plants – but
they draw on
isotopically
different CO2
pools!
Fig. 3.1. 13C distribution in ecosystems. Single arrows indicate CO2 fluxes. The double arrow signifies an equilibrium
isotope fractionation. Numbers for pools indicate 13C values (o/oo) and numbers of arrows indicated the fractionation
(, o/oo) occurring during transfers. Negative 13C values indicate that less heavy isotope is present than in the standard
(which has a 1.1% 13C content; Table 1.2a), not that isotope concentrations are less than zero. From Peterson and Fry
(1987). Reprinted, with permission, from the Annual Review of Ecology and Systematics, Volume 18, copyright 1987 by
Annual Reviews www.annualreviews.org.
From Fry, 2007
Factors affecting isotope fractionation
• Temperature – Affects kinetic (reaction ratedependent) isotope fractionation - Fractionation
decreases with increasing temperature. As total energy of
the system increases (i.e. thermal energy), the fractional
difference between the bond energies of the heavy and
light isotopes becomes less significant.
• Kinetics - heavier isotopes less likely to react
– therefore react slower. (affected by temperature)
• Equilibrium processes – phase changes
reactions (gas/liquid or solute/mineral)
• Diffusion – light isotopes diffuse slightly faster.
Kinetic Isotope Fractionation (depends
on differential rate of reaction for light vs. heavy isotopes)
For reaction sequence of 4 different compounds containing
carbon:
A --> B --> C --> D
If all A is converted to D, then no fractionation will take
place (this is a simple mass balance - if you start with a certain amount
of
13C,
you will finish with the same amount)
If however, only a portion of A is converted to B, and then A
is replenished, then fractionation is likely.
Thus, even if all B is converted to C and all C is converted to
D, fractionation will be evident in D and the fractionation
factor, A -> B will be the same as A-> D
O2 minimum
zone
δ18O
0
7
14
δ18O of O2
Emerson & Hedges, Chap. 5
Equilibrium isotope effects
Equilibrium effects are caused by a preferential enrichment of
one isotope in a crystal lattice site (or mineral phase) relative
to another, based on thermodynamic stability.
Molecules containing the heavy isotope are more stable and
have higher bond dissociation energies. Thus, the heavy
isotope will tend to partition into the solid phases or larger
complexes. This type of equilibrium fractionation is strongly
affected by temperature. This is the basis of stable isotope thermometry!
Likewise, light isotopes will preferentially partition
into the aqueous phase over crystals, or gaseous
phase over aqueous phase.
Example of equilibrium isotope effect –
during glaciations
When seawater evaporates, the
heavy water (H218O) is
preferentially left behind yielding
isotopically heavier (more positive
18O) water and isotopically
lighter H2O vapor.
As water vapor moves through the
atmosphere, precipitation
removes the heavier isotope
preferentially (same principle as
in the evaporation) and the vapor
becomes lighter still.
18O
buildup in ocean
+9o/oo
enrichment
of precip
+9o/oo
enrichment
of precip
Since water vapor transport is generally from tropics to high latitude, snow
deposited at high latitudes has a lighter 18O isotopic composition than
precipitation at lower latitudes. This shifting of the isotope signatures of natural
waters can be used to trace processes such as ice sheet buildup during glacial
periods, paleotemperatures and ocean temperatures.
Different ocean water masses have different isotope
signatures that behave as conservative tracers, aiding in
distinction of mixing patterns in the ocean.
http://earthobservatory.nasa.gov/Study/Paleoclimatology_OxygenBalance/oxygen_balance.html
Heavier
Initial δ
value of
substrate
δ value of
first
products
formed
Little substrate left –
what is left is enriched
in heavy isotope
“Rayleigh Distillation”
For a closed or semiclosed system, the
isotopic composition of
the products and
reactants will depend on
the extent of the
reaction. This is an
example of a “Rayleigh
Distillation”.
From Peterson and Fry, 1986
An example
of a semiclosed system
– sulfate
depletion in a
marine
sediment
Foraminifera (CaCO3, calcite-depositing) preserved in sediments
have proved invaluable in determining paleo conditions in the
ocean, especially temperature and ocean water volume.
Relationship between temperature and the 18O content of
carbonates and water is (Emerson & Hedges Chap 5):
Tcalcite = 17.04 - 4.34(Calcite - water) + 0.16(Calcite - water)2
Forams deposit CaCO3 that is
in isotopic equilibrium with
the seawater. Because each
species has slightly different
fractions factors, it is
necessary to use a single
species of foram in the
analysis.
Benthic and pelagic species
are known
Elphidium excavatum
clavatum
Globigerina sp.
Buccella frigida
Benthic forams
The δ18O in CaCO3 shells reflects the temperature at
which the organisms grew.
Small excursions
of δ18O, but
measurements are
very precise
Shell
Planktonic foram
Benthic foram
Otoliths from Bluefin Tuna show depletion of 13C in response to
changes in Earth’s atmospheric δ 13C
1947
2006
Atmospheric δ13CO2
is going down due to
Suess Effect – input
of fossil carbon with
light isotopic
signature
Several isotopes of N have been used with
utility in the study of nitrogen cycling
14N
is the most abundant stable form of N
15N
is stable and has a natural abundance of 0.365 atom%
13N
is radioactive with a half life of 10 minutes - not very
useful, but it has been used in some studies
Atmospheric N2 is the reference for 15N (i.e. 15Natmos = 0)
Fractionation of N occurs through each level of the food chain,
with each trophic level becoming isotopically heavier (higher 15N).
Phytoplankton fractionate N (take lighter isotope preferentially)
when N is available. When N is severely limiting, fractionation
decreases. Thus, 15N values can tell us something about nutrient
status. Useful for paleo-reconstructions.
Wastewater NO3~ +10 to +20
Fig. 3.2. Representative 15N values in natural systems. See Fig. 1.3a for explanation of symbols. From Peterson and Fry
(1987). Reprinted, with permission, from the Annual Review of Ecology and Systematics, Volume 18, copyright 1987 by
Annual Reviews www.annualreviews.org.
Sigman et al. 2009
Typical del 15N values for marine N pools
Deep ocean nitrate +5 (up to +12 o/oo in denitrification zones)
Atmospheric N
0 o/oo
Phytoplankton
-4 to +8 o/oo
N-fixer biomass
0 o/oo (they draw on atmospheric N2)
Consumers
Variable –trophic enrichment of 15N
along food chain – about 3 o/oo per
δ15N vs. Trophic Position
trophic level
25
δl 15N
20
15
10
5
Primary producers
0
See Karl et al. for data on changes in del 15 N
with changes in Trichodesmium abundance.
0
2
4
Trophic Position
6
15N
enrichment at higher trophic levels on Georges Bank
Sulfur isotopes
Seawater sulfate
+21 o/oo
Sedimentary sulfides (FeS2)
-10 to -40 o/oo
Marine Plankton
+19 o/oo
Spartina alterniflora
-8 to +2 o/oo
Upland plants
+4 to + 6 o/oo
The dissimilatory sulfate reduction process fractionates sulfur
(taking the lighter isotope preferentially) and other
sedimentary sulfur cycle processes further fractionate the
reduced sulfur such that sulfides preserved in sediments are
isotopically light. The large global burial of this “light” sulfur,
explains why the remaining seawater sulfate pool is heavy
(+20 o/oo) compared to the primordial CDT standard.
Fig. 3.3. Representative 34S values in natural systems. See Fig. 1.3a for explanation of symbols. From Peterson and
Fry (1987). Reprinted, with permission, from the Annual Review of Ecology and Systematics, Volume 18, copyright
1987 by Annual Reviews www.annualreviews.org.
Availability of substrate affects
fractionation
“Beggars can’t be choosers”
If substrate is non-limiting (and constantly renewed)
maximum fractionation will take place.
If substrate is limiting (and virtually all is used, with slow
replacement), fractionation will be low
Examples
CO2 limitation of phytoplankton affects
13C
Nitrate availability affects phytoplankton
15N
δ 15N of plankton biomass (o/oo)
+15
+10
Low N availability
– Little
fractionation
Seawater NO3- δ15N value
High N availability –
significant fractionation
+5
0
N-fixing
organisms
5
10
NO3- concentration (μM)
δ 15N values of
plankton
depend on the
source of N
(e.g NO3- vs.
N2) and
availability of
the nutrient.
Isotopes in food web studies
You are what you eat!
• Consumer isotopic composition reflects the isotope
composition of the food source.
• Little fractionation along trophic levels for Carbon or
Sulfur
• Some trophic enrichment of 15N with higher trophic
levels (+3 to +4 o/oo per trophic level) due to
preferential excretion of light isotope.
Typical values for del
13C
Sea water DIC
+2 o/oo
Atmospheric CO2
-7 o/oo
Marine POC
-20 to -22 o/oo
Terrestrial plants
-27 o/oo
Marsh grasses (C4) -14 o/oo
Benthic algae
-17 o/oo
Values for biogenic materials are approximate, and
subject to variation depending on factors such as
temperature and availability of substrates (e.g. CO2)
New data are emerging all the time!
consumers
From Libes, 1992,
Chapter 29.
Original data from
Peterson &
Howarth, 1987
End
Fig. 1.3. You are what you eat stable isotopes in a 50 kg human
who is composed of mostly of
light isotopes with a small
amount of heavy isotopes.
People are mostly water, so
hydrogen and oxygen isotopes
dominate at >35kg along with
carbon isotopes at >11 kg. Then
comes N isotopes. S isotopes are
missing – they should be here at
about 220g for the light isotope
32S and 10g for the heavy
isotope 34S.
Have you had your isotopes
today? (from Wada and Hattori,
1990; reproduced with
permission of CRC Press LLC).
From Fry, Stable Isotope
Ecology, 2006
If I ate only
marine fish I
would have
138.1 g of
13C
O2
consumption
results in an
O2 pool which
is isotopically
heavier
because
“light” O2 is
used
preferentially
In H2O
Bacterial enzymes will show a slight preference for the
light isotope of reactants, yielding isotopically light
products.
Consider a steady state pool of glucose in seawater
(production = consumption). Given the unchanging reservoir
Bad example because
of glucose carbon, fractionation can take place. If the 13C
carbon is not
of carbon in glucose is -18 o/oo, then the 13C of CO2
subtantially
produced from respiration of this glucose carbon may be -30
fractionated in
o/oo (preference for light isotope). By mass balance, the residual
respiration
steady-state glucose pool will have a slightly heavier 13C
than the source glucose entering the pool (say from phyto
exudate)
Oxygen consumed in this respiration reaction may also be
fractionated. The enzymes will slightly prefer the 16O,
leaving the residual O2 pool isotopically heavier.
Biogenic CH4 can be distinguished isotopically from
thermogenic CH4. Why?
Kinetic vs. equilibrium isotope effects
Kinetic isotope effects are common both in nature and in the
laboratory and their magnitudes are generally much larger than
those of equilibrium isotope effects.
Kinetic isotope effects are normally associated with fast,
incomplete, or unidirectional processes like evaporation,
diffusion, dissociation reactions and biological “enzymatic”
reactions. The examples of diffusion and evaporation are
explained by the different rates of reaction (i.e. phase
transfer) by the different isotopic forms of molecules as they
move through a phase or across a phase boundary.
Examples of kinetic effects include evaporation, leaf
respiration, bacterial respiration.
This is Redundant
Example of equilibrium isotope effect
Isotopic exchange
H216O + C18O16O(aq) <=> H218O + C16O16O(aq)
HC16O16O16O- + H218O <=> HC16O16O18O- + H216O
(bicarbonate)
In seawater the total DIC pool is in isotopic equilibrium with
water
At low temperature, more 18O partitions into HCO3- therefore
at low temperature CaCO3 is enriched in 18O (i.e. It becomes
isotopically heavier)
These are only fractional enrichments! Not all
will partition into CO32-.
18O
This is too long – cut out some of the
Kinetic fractionation stuff
• Organize better.
• Focus more on applications
• Focus on the food web stuff. Find a new
paper that uses multiple stable isotopes in a
food web study.
• Develop some problems to work on for HW
Isotope composition of natural material differs
CO2
in
air
SW
DIC
-30
-20
-10
0
-30
-20
-10
0
-30
-20
-10
0
Why do these isotopic variations exist?
Closed system vs. open system
Much water in clouds originates from evaporation of ocean
water in tropics. Cloud condensation reactions – tend to
enrich cloud water in lighter isotopes (i.e. 16O), leaving heavy
isotopes (i.e. 18O) behind in the ocean. Poleward transport
of cloud water, and eventual deposition of this on polar
regions where it forms ice caps, leads to build up of
isotopically light water in the ice, and isotopically heavy
water in the oceans. During glacial ice build-up, the oceans
become “heavier”. This record is preserved in marine
carbonates and other paleoindicators.
Isotopic differences in materials are measured relative
to a standard material.
Standards used for different stable isotopes
Element
Standard
Comments
Carbon
Oxygen
Pee Dee Belemnite
(PDB)
PDB fossil carbonate has long since
been used up. New standards such as
NB-20 are used, and directly related
to PDB
Oxygen
Hydrogen
Standard Mean
Ocean Water
(SMOW)
Ocean water is largest reservoir of
water on the planet. Good reference.
Nitrogen
Atmospheric
Nitrogen
Isotopic composition of the
atmosphere is very constant, therefore
a good reference
Sulfur
Canyon Diablo
Troilite (CDT)
The CDT is a meteor, therefore it
represents “primordial” sulfur
From Libes, 1992, Chapter 29
From Broecker, 1997
Interglacial period (warm)
Glacial period (cold)
YD- 1000y cool
period within warm
Fractionation factor for carbon in photosynthesis is the same for marine and
terrestrial plants – but they draw on isotopically different CO2 pools!
δ15N vs. Trophic Position
25
δl 15N
20
15
10
5
0
0
1
2
3
Trophic Position
4
5
6