Lecture 35
advertisement

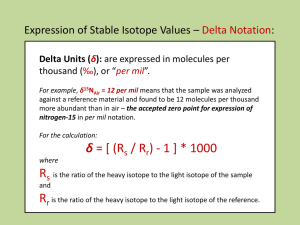
Stable Isotope Geochemistry: Theory Lecture 35 Fractionations • Isotope fractionation can originate from both kinetic effects and equilibrium effects. • Equilibrium Fractionations: o Quantum mechanics predicts that the mass of an atom affects its vibrational motion, and therefore the strength of chemical bonds. It also affects rotational and translational motions. From an understanding of these effects of atomic mass, it is possible to predict the small differences in the chemical properties of isotopes quite accurately. • Kinetic Fractionations o Lighter isotopes form weaker bonds and therefore react faster. They also diffuse more rapidly. These also lead to isotopic differences. Equilibrium Fractionations • • • • • Equilibrium fractionations arise from translational, rotational and vibrational motions of molecules in gases and liquids and atoms in crystals. Isotopes will be distributed so as to minimize the vibrational, rotational, and translational energy of a system. All these motions are quantized (but quantum steps in translation are very small). Of the three types of energies, vibrational energy makes by far the most important contribution to isotopic fractionation. Vibrational motion is the only mode of motion available to atoms in a solid. These effects are small. For example, the equilibrium constant for the reaction: ½C16O2 + H218O ⇄ ½C18O2 + H216O is only about 1.04 at 25°C and the ∆G of the reaction, given by -RT ln K, is only -100 J/mol Predicting Fractionations • Boltzmann distribution law states that the probability of a molecule having internal energy Ei is: gi e- Ei /kT Pi = å gne- Ei /kT n o g is a weighting factor to account for degenerate states. • The denominator is the partition function: Q = å gn e- Ei /kT n • From partition functions we can calculate the equilibrium constant: K = Õ Qini i • We can divide the partition function into three parts: Qtotal = QtransQrotQvib o The separate partition functions can be calculated separately. Partition Functions • The following applies to diatomic molecules, for which the sums in the partition function fortunately have simple solutions. Principles are the same for multiatomic molecules and crystals, but the equations are more complex because there are many Note error in book: possible vibrations and rotations. remove 2 in • Vibrational Partition Function: - hn /2kT Qvib = o e 1- e- hn /kT exponential term in denominator where h is planks constant (converting frequency to energy) and ν is the vibrational frequency of the bond. • Rotational Partition Function 8p 2 IkT Qrot = s h2 o where I is the moment of inertia I = µr2; i.e., the reduced mass (µ) of atoms times bond length.σ is a symmetry factor; σ= 1 for a non-symmetric molecule (18O16O) and 2 for a symmetric one (16O16O) • Translational partition function for molecule of mass m is (derived from Schrödinger’s equation for particle in a box): Qtrans (2p mkT )3/2 = V h3 Partition Function Ratios K = Õ Qin • Since i • what we really want is the ratios of partition functions for isotope exchange reactions. Most terms cancel (including bond length). • This ratio for two isotopic species (isotopologues) of the same diatomic molecules, e.g., 16O18O and 16O16O, will be: i - hn Q16 O 18 O Q16 O 2 hn 16 O 18 O e 16 O 18 O 3/2 - hn 16 /kT -hn 16 18 /2 kT H O - hn 16 18 /kT µ16 O 18 O m16 O 18 O )µ16 O 18 Os 16 O m163/2O 18 O s 16 O 18 O kT 1- e O O n 16 O 18 O e O O (1- e 2 2 = = - hn 16 /2 kT - hn 16 /2 kT - hn 16 18 /kT O O 2 2 hn 16 O e n 16 O e (1- e O O )µ16 O s 16 O 18 O m163/2O 3/2 2 2 2 2 µ16 m16 s 16 O kT 1- e-hn 16 O2 /kT O2 O2 2 /2 kT • We see the partition function ratio is temperature dependent (which arises only from the vibrational contribution: temperature canceled in other modes). • We also see that we can predict fractionations from measured vibrational frequencies and atomic and molecular masses. Temperature Dependence • • • • The temperature dependence is: e- hn /2kT Qvib = 1- e- hn /kT At low-T (~surface T and below), the exponential term is small and the denominator approximates to 1. Hence hn - hn /2kT Qvib » e = 1- a = A+ • O isotope fractionation between CO2 and H2O B T At higher temperature, however, this approximation no longer holds and α varies with the inverse square of T: a = A+ • 2kT and the fractionation factor can be expressed as B T2 The temperature dependence leads to important applications in geothermometry & paleoclimatology Kinetic Fractionations: Reaction Rates • • • • Looking again at the hydrogen molecular bond, we see it takes less energy to break if it is H-H rather than D-H. This effectively means the activation energy is lower and the rate constant, k, will be higher: DH will react faster than H2. We can calculate a kinetic fractionation factor from the ratio of rate constants: kD e-(e -1/2hn D )/kT a= = kH e -(e -1/2hn H )/kT This will make no difference if the reaction goes to completion, but will make a difference for incomplete reactions. (good example is photosynthesis, which does not convert all CO2 to organic carbon). Kinetic Fractionation: Diffusion • Lighter isotopic species will diffuse more rapidly. o Energy is equally partitioned in a gas (or liquid). The translational kinetic energy is simply E = ½mv2. o Consider two molecules of carbon dioxide, 12C16O2 and 13C16O2, in a gas. If their energies are equal, the ratio of their velocities is (45/44)1/2, or 1.011. Thus 12C16O2 can diffuse 1.1% further in a given amount of time at a given temperature than 13C16O2. But this applies to ideal gases (i.e., low pressures where collisions between molecules are infrequent. o For the case of air, where molecular collisions are important, the ratio of the diffusion coefficients of the two CO2 species is the ratio of the square roots of the reduced masses of CO2 and air (mean molecular weight 28.8): µ12 CO -air 4.1906 D12 CO o 2 2 = = = 1.0044 D13CO µ13CO -air 4.1721 2 2 o leading to a 4.4‰ fractionation (actually observed). Rayleigh Distillation/Condensation • • Different isotopologues of water evaporate at different rates and have different condensation temperatures. We can imagine two ways in which condensation occurs: o o • • droplets remain in isotopic equilibrium with vapor droplets do not remain in equilibrium: fractional condensation If the fractionation between vapor and liquid is α, for fractional condensation, the fractionation, ∆, varies with fraction of vapor remaining, ƒ, as: ∆ = 1000(ƒα-1-1) For equilibrium condensation it is: æ ö 1 ∆ = ç 1´1000 è (1- f ) / a + ƒ ÷ø • Fractional condensation can lead to quite extreme compositions of remaining vapor. Isotopic composition of vapor when the fraction of original vapor, ƒ, remains. Isotope Fractionations • As a rule, heavy isotopes partition preferentially into phases in which they are most strongly bound (because this results in the greatest reduction in system energy). o Covalent bonds, and bonds to heavier atoms, are generally strongest and hence will most often incorporate the heavy isotope. • Largest fractionations will occur where the atomic environment or bond energy differences are greatest o So, for example, fractionation of O between silicates are not large, because the O is mainly bound to Si. • Fractionations tend to be large between different oxidation states of an element (e.g., for C, N, S). • Lighter isotopes are likely to be enriched in the products of incomplete reactions and also reactions where diffusion is important. Mass Dependent Fractionation • • • • • if a 4‰ fractionation of δ18O is observed in a particular sample, what value of δ17O do we predict? We might guess it would ½ as much. Mass occurs in a variety of ways in the partition function, as m3/2, as reduced mass, and in the exponential term. Consequently, the ratio of fractionation of 17O/16O to that of 18O/16O in most cases is about 0.52. Nevertheless, the fractionation between isotopes predicted by this equation is proportional to the difference in mass – this is referred to as mass-dependent fractionation. There are some exceptions where the ratio of fractionation of 17O/16O to that of 18O/16O is ≈1. Since the extent of fractionation in these cases seems independent of the mass difference, this is called mass-independent fractionation. Examples o o o Oxygen in meteorites Sulfur in Archean sulfides Oxygen in stratospheric gases Mass Independent Fractionation • • • • • • Most examples of ‘MIF’ seem to be related to photochemical reactions. Formation of ozone in the stratosphere involves the energetic collision of monatomic and molecular oxygen: O + O2 → O3 The ozone molecule is in a vibrationally excited state and subject to dissociation if it cannot lose this excess energy. The excess vibrational energy can be lost either by collisions with other molecules, or by partitioning to rotational energy. In the stratosphere, collisions are infrequent, hence repartitioning of vibrational energy represents an important pathway to stability. Because there are more possible energy transitions for asymmetric species such as 16O16O18O and 16O16O17O than symmetric ones such as 16O16O16O, the former can more readily repartition its excess energy and form a stable molecule. In the troposphere, collisions more frequent, reducing this effect. Isotope Geothermometry • One of the principal uses of stable isotopes is geothermometry. Stable isotope geothermometers are based on the temperature dependence of the fractionation factor or equilibrium constant, which can generally be expressed as: a = A+ o • B T2 (at low temperatures, the form of changes to α ∝ 1/T). Temperature dependence can be theoretically calculated or experimentally measured. Measuring the isotopic composition of two phases allows us to calculate the temperature at which they equilibrated (assuming, of course, that they did equilibrate). Isotope “Clumping” • Consider the distribution of 18O between CO and O2 (Example 9.1). CO and O2 will consist actually of 12 isotopically distinct molecules or “isotopologues”, such as 12C16O, 12C17O, 13C18O, 16O17O, etc. • The distribution of isotopes between these species will not be random but rather some of these isotopologues will be thermodynamically favored. o Essentially, grouping the heavy isotopes in one molecule, e.g., 13C18O, reduces bond energy by a bit more than twice the reduction of putting one heavy isotope in the molecule. Thus “clumping” of heavy isotopes reduces system energy. • This ‘clumping’ depends on temperature (greater at low T). By analyzing the various isotopologues of the species, one can calculate equilibrium temperatures. o o The advantage is that we need analyze just one phase involved in the reaction, for example, carbonate precipitated from water. In addition to calculating temperature, one can also calculate the isotopic composition of the water. • This is a very new field, but holds great promise in isotope geothermometry.