Organic Chemistry
advertisement
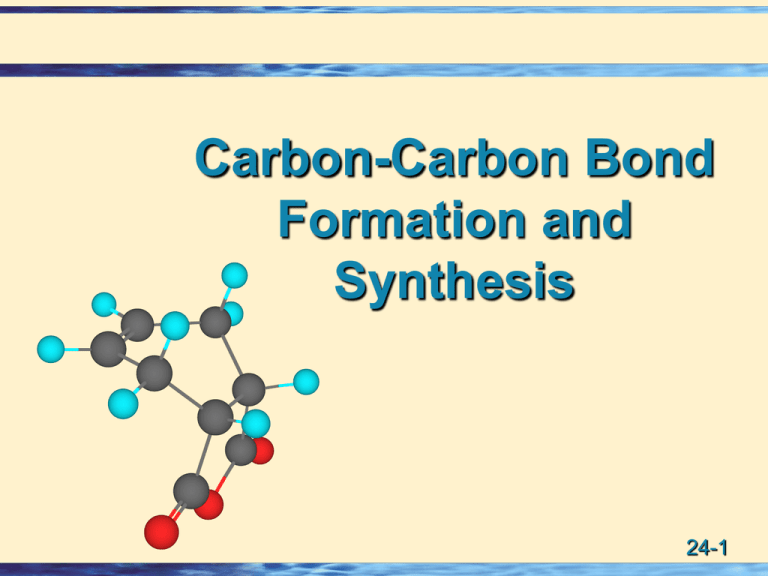
Carbon-Carbon Bond Formation and Synthesis 24-1 Organometallic Compounds Recall: two extremely important reactions of metals and organometallic compounds: • Oxidative addition: The addition of a reagent to a metal center causing it to add two substituents which extract two electrons from the metal and increasing its oxidation state by two. • Reductive elimination: The elimination of two substituents which donate two electrons to the metal center causing the oxidation state of the metal to decrease by two. oxid ative add ition X MLn + X2 MLn reductive X elimin ation 24-2 Heck Reaction Overall: A palladium-catalyzed reaction in which the R group of RX, a haloalkene or haloarene, is substituted for a vinylic H of an alkene. H R-X + Haloalk ene Alken e or Haloarene + B Pd catalys t Heck reaction Base R + Subs tituted alken e BH+ X- Conjugate acid of the b ase 24-3 Heck Reaction (consider the alkene) • Substitution (H R) is highly regioselective; most commonly at the less substituted carbon of the double bond. • Substitution is highly stereoselective; the E configuration is often formed almost exclusively. O Br Bromobenzen e + CH2 =CHCOCH3 Meth yl 2-p ropen oate (Methyl acrylate) Pd catalyst Heck reaction E O COCH3 Less substituted, H Ph substitution occurs here. Neither E nor Z Methyl (E)-3-phen yl-2-p ropenoate (Meth yl cinnamate) 24-4 Heck Reaction (RX = Haloalkene) • For RX = haloalkene: Reaction is stereospecific; the configuration of the double bond in the haloalkene is E preserved. Z Z I + (Z)-3-Iodo-3hexen e Ph Styren e Ph Pd catalys t Heck reaction (1E,3Z)-1-Phen yl-3ethyl-1,3-h exadiene E E I (E)-3-Iod o-3hexen e + Ph Styren e Ph E Pd catalys t Heck reaction (1E,3E)-1-Phenyl-3ethyl-1,3-h exadiene 24-5 Heck Reaction. Some considerations. The catalyst: • • • • most commonly Pd(II) acetate. reduced in situ to Pd(0). reaction of Pd(0) with good ligands gives PdL2. The organic halogen compound: aryl, heterocyclic, benzylic, and vinylic iodides, chlorides, bromides, and triflates (CF3SO2O-). • alkyl halides with an easily eliminated b hydrogen are rarely used because they undergo b-elimination to give alkenes. • OH groups and the C=O groups of aldehydes, ketones, and esters are unreactive under Heck conditions. 24-6 Heck Reaction. More… The alkene • The less the crowding on the alkene, the more reactive it is. The base • Triethylamine, sodium, and potassium acetate, and sodium hydrogen carbonate are most common The solvent. • Polar aprotic solvents such as DMF, acetonitrile, and DMSO. • aqueous methanol may also be used. The ligand • Triphenylphosphine, PPh3, is one of the most common. 24-7 Heck Reaction Start here L = PPh3 R- X oxidati ve BH X X addi ti on R L 2 Pd L 2 Pd R4 H 1 R B: 0 HX II 5 redu cti ve 2 R3 R2 s yn e li mi n ati on addi ti on Th e catalytic X cycl e of th e X II L 2 Pd II He ck reacti on R L 2 Pd + - H 4 R4 R2 R4 s yn e li mi n ati on X II L 2 Pd R3 H R2 3 rotati on about righ tthe abou t bond. Rotation C-C the C -C the bonRd is swapped This is where H in, replacing the H. R3 R R4 R3 R R2 24-8 Heck Reaction • The usual pattern of acyclic compounds: replacement of a hydrogen of the double bond with the R group. • If the R group has no H for syn elimination, then a b H may be abstracted elsewhere. I + This b H should be brought into position for syn elimination with the Pd. Can’t happen due to cyclohexane ring. Pd(OAc) 2 (C 2 H5 ) 3 N H PdL2 OAc H H (racemic) 24-9 Suzuki Coupling Suzuki coupling: A palladium-catalyzed reaction of an organoborane (R’-BY2) or organoborate (RB(OMe)2) with an alkenyl, aryl, or alkynyl halide, or triflate (R-X) to yield R-R’. Overall: R'-BY2 + R-X Organob oron Compounds B RCH=CH B Alkyl B PdL4 , Base R-R' + XBY2 Cou pling reagents X = h alide or triflate) X RCH=CHX RC C X Alkyl-X (D ifficult) 24-10 Suzuki Coupling • Recall boranes are easily prepared from alkenes or alkynes by hydroboration. H + ( Sia) 2 BH hydroboration B(Sia) 2 H • Borates are prepared from alkyl or aryl lithium compounds and trimethylborate. Li + B(OMe) 3 B(OMe) 2 + LiOMe PhCl + Li 24-11 Suzuki Coupling • These examples illustrate the versatility of the reaction. H H B(Sia) 2 + Br C6 H1 3 H B(OMe) 2 + Br NH2 N B( OMe) 2 Pd( Ph3 ) 4 NaOMe C6 H1 3 H Ph( OAc) 2 Na2 CO3 NH2 N Pd( OAc) 2 + Br Et 3 N 24-12 Suzuki Coupling Reductive elimination Oxid. Addn Transmetalation R1 and OtBu swap Substitution 24-13 Alkene Metathesis Alkene metathesis: A reaction in which two alkenes interchange carbons on their double bonds. A A B A B catalys t + A A A B B A A + B B B B • If the reaction involves 2,2-disubstituted alkenes, ethylene is lost to give a single alkene product. A A B H A catalys t + H A B H H + B CH2=CH2 B 24-14 Alkene Metathesis • A useful variant of this reaction uses a starting material in which both alkenes are in the same molecule, and the product is a cycloalkene. EtOOC COOEt EtOOC catalys t COOEt + CH2 = CH2 • Catalysts for these reactions are a class of compounds called stable nucleophilic carbenes. 24-15 Stable Nucleophilic Carbenes • Carbenes and carbenoids provide the best route to three membered carbon rings. • Most carbenes are highly reactive electrophiles. • Carbenes with strongly electron-donating atoms, however, for example nitrogen atoms, are particularly stable. • Rather than being electron deficient, these carbenes are nucleophiles because of the strong electron donation by the nitrogens. • Because they donate electrons well, they are excellent ligands (resembling phosphines) for certain transition metals. • The next screen shows a stable nucleophilic carbene. 24-16 Nucleophilic Carbene • A stable nucleophilic carbene. N N N – N + N – N+ 24-17 Alkene Metathesis Catalyst • A useful alkene methathesis catalyst consists of ruthenium, Ru, complexed with nucleophilic carbenes and another carbenoid ligand. • In this example, the other carbenoid ligand is a benzylidene group. R N n u cle ophi l ic carbe n e s N R Cl Cl R N Ru C6H5 N R 24-18 Ring-Closing Alkene Metathesis Like the Heck reaction, alkene metathesis involves a catalytic cycle: • Addition of a metallocarbenoid to the alkene gives a four-membered ring. • Elimination of an alkene in the opposite direction gives a new alkene. 24-19 Ring-Closing Alkene Metathesis 24-20 Ring-Closing Alkene Metathesis Initiation Step Cycle start 24-21 Diels-Alder Reaction Diels-Alder reaction: A cycloaddition reaction of a conjugated diene and certain types of double and triple bonds. • dienophile: Diene-loving. • Diels-Alder adduct: The product of a Diels-Alder reaction. O O + 1,3-Butadie n e 3-Bu te n -2-on e (a die n e ) (a die n oph i le ) Di e ls -A lde r addu ct 24-22 Diels-Alder Reaction • Alkynes also function as dienophiles. COOEt COOEt + COOEt 1,3-bu tadi e n e Di eth yl (a die n e ) 2-bu tyn e dioate (a die n oph i le ) COOEt Di el s -Al der addu ct • Cycloaddition reaction: A reaction in which two reactants add together in a single step to form a cyclic product. 24-23 Diels-Alder Reaction • We write a Diels-Alder reaction in the following way: Di en e Di en oph il e Addu ct • The special value of D-A reactions are that they: 1. form six-membered rings. 2. form two new C-C bonds at the same time. 3. are stereospecific and regioselective. Note the reaction of butadiene and ethylene gives only traces of cyclohexene. 24-24 Diels-Alder Reaction • The conformation of the diene must be s-cis. s-t rans conformation (lower in energy) s-cis conformation (higher in energy) 24-25 Diels-Alder Reaction Steric Restrictions • (2Z,4Z)-2,4-Hexadiene is unreactive in Diels-Alder reactions because nonbonded interactions prevent it from assuming the planar s-cis conformation. methyl grou ps forced clos er th an allow ed by van der Waals radii s-t rans conformation s-cis conformation (low er energy) (h igh er energy) (2Z,4Z)-2,4-Hexadiene 24-26 Diels-Alder Reaction • Reaction is facilitated by a combination of electronwithdrawing substituents on one reactant and electron-releasing substituents on the other. 200°C pre s s u re + 1,3-Bu tadie n e Eth yl en e C ycloh e xen e O O 140°C + 1,3-Bu tadie n e 3-Bu te n -2-on e O O + 2,3-Di me th yl - 3-Bu te n -2-on e 1,3-bu tadie n e 30°C 24-27 Diels-Alder Reaction Ele ctron -Re l e as i n g Grou ps Ele ctron -W i th drawi n g Grou ps - CH 3 , alkyl group s - CH O (ald ehyde, ketone) - OR (ether) - COOH (carb oxyl) - OOCR (es ter) - COOR (es ter) - NO 2 (nitro) -C N (cyano) 24-28 Diels-Alder Reaction • The Diels-Alder reaction can be used to form bicyclic systems. + room tem peratu re Di en e Di en oph i le 170°C H H Di cyclope n tadie n e (en do form ) 24-29 Diels-Alder Reaction • Exo and endo are relative to the double bond derived from the diene. the dou bl e bon d de ri ve d from the di e n e e xo (ou tsi de) e n do (i n si de ) rel ati ve to the dou bl e bon d 24-30 Diels-Alder Reaction • For a Diels-Alder reaction under kinetic control, endo orientation of the dienophile is favored. O + Cyclopentadiene OCH3 Methyl propenoate 7 COOCH 3 H redraw 6 5 4 1 2 3 COOCH 3 Me th yl bi cyclo[2.2.1]h e pt-5-e n e n do-2-carboxylate (racem ic) 24-31 Diels-Alder Reaction • The configuration of the dienophile is retained. COOCH 3 COOCH 3 + COOCH 3 A cis die n oph il e) H3 COOC COOCH 3 Di me th yl cis-4-cycl oh exe n e 1,2-di carboxylate COOCH 3 + COOCH 3 A trans die n oph il e) COOCH 3 Di me th yl trans- 4-cycl oh exe n e 1,2-di carboxylate (racem ic) 24-32 Diels-Alder Reaction • The configuration of the diene is retained. CH3 O + H3 C O CH3 H3 C CH3 O H3 C H O H O O O CH3 O O O O + H H3 C H O 24-33 Diels-Alder Reaction Mechanism • No evidence for the participation of either radical of ionic intermediates. • Chemists propose that the Diels-Alder reaction is a concerted pericyclic reaction. Pericyclic reaction: A reaction that takes place in a single step, without intermediates, and involves a cyclic redistribution of bonding electrons. Concerted reaction: All bond making and bond breaking occurs simultaneously. 24-34 Diels-Alder Reaction • Mechanism of the Diels-Alder reaction 24-35 Aromatic Transition States Hückel criteria for aromaticity: The presence of (4n + 2) pi electrons in a ring that is planar and fully conjugated. Just as aromaticity imparts a special stability to certain types of molecules and ions, the presence of (4n + 2) electrons in a cyclic transition state imparts a special stability to certain types of transition states. • Reactions involving 2, 6, 10, 14.... electrons in a cyclic transition state have especially low activation energies and take place particularly readily. 24-36 Aromatic Transition States • Decarboxylation of b-keto acids and b-dicarboxylic acids. O H O H O O C O (A cycl ic s i x-me mbe re d tran s i ti on s tate ) O + CO 2 O e n ol of a k e ton e • Cope elimination of amine N-oxides. C H C CH3 heat N+ O CH3 A cyclic six -membered transition state CH3 C C An alkene + H N O CH3 N,N-dimethylhydrox ylamine 24-37 Aromatic Transition States • the Diels-Alder reaction Di en e Di en oph il e Addu ct • pyrolysis of esters (Problem 22.42) We now look at examples of two more reactions that proceed by aromatic transition states: • Claisen rearrangement. • Cope rearrangement. 24-38 Claisen Rearrangement Claisen rearrangement: A thermal rearrangement of allyl phenyl ethers to 2-allylphenols. O OH 200-250°C Allyl ph enyl ether 2-A llylp henol 24-39 Claisen Rearrangement O O h e at Al lyl ph en yl e th e r Tran s ition s tate OH O k e to-e n ol tau tome ri s m H A cycloh e xadi en on e i n te rme diate o-Al lyl ph en ol 24-40 Cope Rearrangement Cope rearrangement: A thermal isomerization of 1,5-dienes. heat 3,3-Dime th yl1,5-he xadie n e 2-Meth yl-2,6h e ptadi e n e 24-41 Cope Rearrangement Example 24.8 Predict the product of these Cope rearrangements. 350°C (a) OH 320°C (b) H 24-42 Synthesis of Single Enantiomers • We have stressed throughout the text that the synthesis of chiral products from achiral starting materials and under achiral reaction conditions of necessity gives enantiomers as a racemic mixture. • Nature achieves the synthesis of single enantiomers by using enzymes, which create a chiral environment in which reaction takes place. • Enzymes show high enantiomeric and diastereomeric selectivity with the result that enzyme-catalyzed reactions invariably give only one of all possible stereoisomers. 24-43 Synthesis of Single Enantiomers How do chemists achieve the synthesis of single enantiomers? The most common method is to produce a racemic mixture and then resolve it. How? • the different physical properties of diastereomeric salts. • the use of enzymes as resolving agents. • chromatographic on a chiral substrate. 24-44 Synthesis of Single Enantiomers • In a second strategy, asymmetric induction, the achiral starting material is placed in a chiral environment by reacting it with a chiral auxiliary. Later it will be removed. • E. J. Corey used this chiral auxiliary to direct an asymmetric Diels-Alder reaction. • 8-Phenylmenthol was prepared from naturally occurring enantiomerically pure menthol. Me HO Me several step s Me Menth ol (enan tiomerically p ure) Ph Me Me HO Me 8-Phen ylmenthol (an enan tiomerically pure chiral auxillary) 24-45 Synthesis of Single Enantiomers • The initial step in Corey’s prostaglandin synthesis was a Diels-Alder reaction. • By binding the achiral acrylate with enantiomerically pure 8-phenylmenthol, he thus placed the dienophile in a chiral environment. • The result is an enantioselective synthesis. Me OBn Ph Me O + O Achiral Me Enantiomerically pure D iels-A lder 89% BnO OBn + O 97% OR RO O 3% 24-46 Synthesis of Single Enantiomers • A third strategy is to begin a synthesis with an enantiomerically pure starting material. • Gilbert Stork began his prostaglandin synthesis with the naturally occurring, enantiomerically pure Derythrose. • This four-carbon building block has the R configuration at each stereocenter. • With these two stereocenters thus established, he then used well understood reactions to synthesize his target molecule in enantiomerically pure form. OH O HO H OH D-Erythrose 24-47




![[A] t - Dr. Agus Setiabudi, M.Si.](http://s2.studylib.net/store/data/005634279_1-eed3e2d8492eb37cb8d8ed06da0883dc-300x300.png)