Organic Chemistry Fifth Edition
advertisement

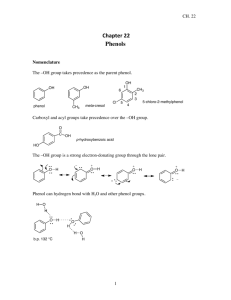
Phenols Infrared Spectrum of p-Cresol Proton NMR H H HO CH3 H 10.0 9.0 8.0 7.0 6.0 H 5.0 4.0 3.0 Chemical shift (, ppm) 2.0 1.0 0 13C NMR OH 128.5 OCH3 155.1 121.1 159.7 115.5 114.0 129.8 129.5 120.7 Oxygen of hydroxyl group deshields carbon to which it is directly attached. The most shielded carbons of the ring are those that are ortho and para to the oxygen. Mass Spectrometry Prominent peak for molecular ion. Most intense peak in phenol is for molecular ion. •+ OH •• m/z 94 Physical Properties The OH group of phenols allows hydrogen bonding to other phenol molecules and to water. Hydrogen Bonding in Phenols O H O Physical Properties C6H5CH3 C6H5OH C6H5F Molecular weight 92 94 96 Melting point (°C) –95 43 –41 Boiling point (°C,1 atm) 111 132 85 Solubility in H2O (g/100 mL,25°C) 0.05 8.2 0.2 Acidity of Phenols Their most characteristic property Compare •• •• O •• – •• O •• H pKa = 10 H pKa = 16 •• CH3CH2O •• H + + •• – + + CH CH O • H 3 2 • •• Delocalized negative charge in phenoxide ion – •• •• O •• •• •• O H H H H H H H – •• H H H Delocalized negative charge in phenoxide ion •• •• •• O H H •• – H •• O H H H H – •• H H H Delocalized negative charge in phenoxide ion •• •• •• O H H •• – H •• O H H H H – H •• H H Phenols are converted to phenoxide ions in aqueous base •• •• O •• – •• O •• H – + HO stronger acid + H2O weaker acid Substituent Effects on the Acidity of Phenols Question Which one of the following has the lowest pKa? A) B) C) D) Electron-releasing groups have little or no effect OH pKa: 10 OH OH CH3 OCH3 10.3 10.2 Electron-withdrawing groups increase acidity OH pKa: 10 OH OH Cl NO2 9.4 7.2 Effect of electron-withdrawing groups is most pronounced at ortho and para positions OH OH OH NO2 NO2 NO2 pKa: 7.2 8.4 7.2 Effect of strong electron-withdrawing groups is cumulative OH OH OH NO2 NO2 pKa: 7.2 NO2 4.0 NO2 O2N NO2 0.4 Picric acid Resonance – •• •• O •• •• •• O •• H H H H H H H H •• O •• N + •• O •• •• – •• •• O – •• N + •• O •• •• – Question Which of the following compounds is more acidic? A) o-Cresol B) o-Chlorophenol C) o-Methoxyphenol D) o-nitrophenol E) m-nitrophenol Preparation of Aryl Ethers Typical Preparation is by Williamson Synthesis ONa + RX SN2 OR + NaX but the other combination X + RONa fails because aryl halides are normally unreactive toward nucleophilic substitution Example OH K2CO3 + H2C CHCH2Br acetone, heat OCH2CH CH2 (86%) Aryl Ethers from Aryl Halides F OCH3 + KOCH3 NO2 CH3OH + KF 25°C NO2 (93%) nucleophilic aromatic substitution is effective with nitro-substituted (ortho and/or para) aryl halides Claisen Rearrangement of Allyl Aryl Ethers Allyl Aryl Ethers Rearrange on Heating OCH2CH CH2 200°C OH CH2CH (73%) CH2 allyl group migrates to ortho position Mechanism OCH2CH CH2 O rewrite as OH keto-to-enol isomerization O H Sigmatropic Rearrangement Claisen rearrangement is an example of a sigmatropic rearrangement. A bond migrates from one end of a conjugated electron system to the other. this bond breaks O O “conjugated electron system” is the allyl group H this bond forms Question What will be the Claisen rearrangement product of the carbon-14 labeled ether shown here? A) B) C) D) Oxidation of Phenols: Quinones Quinones The most common examples of phenol oxidations are the oxidations of 1,2- and 1,4-benzenediols to give quinones. OH O Na2Cr2O7, H2SO4 H2O OH O (76-81%) Quinones The most common examples of phenol oxidations are the oxidations of 1,2- and 1,4-benzenediols to give quinones. OH O OH O Ag2O diethyl ether CH3 CH3 (68%) Some quinones are dyes O OH OH O Alizarin (red pigment) UV-VIS Oxygen substitution on ring shifts max to longer wavelength; effect is greater in phenoxide ion. OH max 204 nm 256 nm max 210 nm 270 nm O max 235 nm 287 nm – Some quinones are important biomolecules O CH3 CH3 O CH3 CH3 CH3 Vitamin K (blood-clotting factor) CH3 Some quinone precursors are important in foods Antioxidants can protect against the celldamaging effects of free radicals. Some dietary phenolics are oxidized to quinones as free radical-antioxidant traps. Eg. Reservatrol / Flavonoids