Unkwn2 - The University of Illinois Archives
advertisement
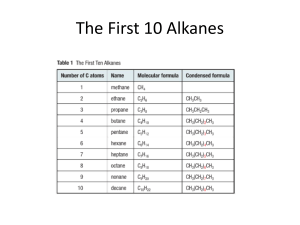
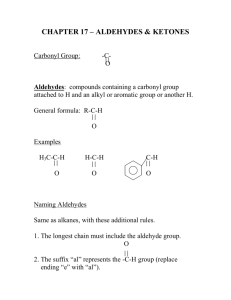
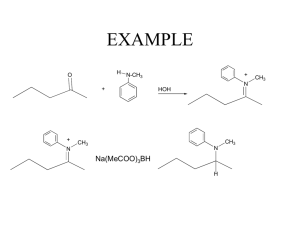
Identify an Unknown Type of compound: Aldehyde Alcohol Amine Ketone Procedure 1. Physical Properties Melting point or boiling point 2. Functional Group Infrared spectrum NMR Spectrum Solubility Classification Tests 3. Solid Derivative Measure boiling point of liquids Distill your unknown and note the boiling point If can’t distill or not enough sample use this technique. Functional Group Carbonyl Group (1650 - 1725 cm-1)? No Yes Alcohol Amine Aldehyde Ketone Broad OH in IR NMR + - Aldehyde Ketone Yes Alcohol No Amine (Basic?) 3700 - 4000 cm-1 Yes Primary or Secondary No Tertiary 2,4-dinitrophenylhydrazine test NH2NH NO2 NO2 Aldehyde or ketone 2,4-dinitrophenylhydrazone O + R-C-R NH2NH NO2 NO2 R C R NO2 NNH NO2 DNP Mechanism OH O C NH2R C C NR NHR Iodoform Test Reagent: NaOH and I2 (NaOI) O RCCH3 I2, NaOH RCOOH + CHI 3 Yellow Iodoform Test O RCCH3 I2, NaOH RCOOH + CHI 3 Yellow OH RCHCH3 I2, NaOH RCOOH + CHI 3 Yellow Iodoform Test Ceric Nitrate Test for Alcohols (NH4)2Ce(NO3)6 + ROH (NH4)2Ce(NO3)5OR + HNO3 Amines 1. Odor 2. If not soluble in water they may dissolve in dilute aqueous acid (HCl). 3. Water solutions of amines are basic to litmus. Hinsberg Test for Amines SO2Cl Benzenesulfonyl Chloride Hinsberg Test for Amines Primary: Soluble. PPT if add HCl Secondary: Insoluble Tertiary: Tends not to react Derivatives Aldehydes and Ketones 1. 2,4-dinitrophenylhydrazone 2. Semicarbazone O O O NH2NHCNH2 + RC R semicarbazide R C NNHCNH2 R semicarbazone Alcohol Derivative O2N O2N O O ROH C Cl O2N 3,5-dinitrobenzoyl chloride C OR O2N 3,5-dinitrobenzoate Amine Derivatives Primary and Secondary Amines O O C Cl + RNH 2 C NHR Benzoyl Chloride SO2Cl + RNH 2 Benzenesulfonyl Chloride Benzamide SO2NHR Benzenesulfamide Sample Unknown B.p. =198-200o DNP = 231-235o Table Structure of Unknown O C acetophenone CH3 Sample Unknown B.p. = 80 - 85o 3,5-dinitrobenzoate = 119 - 121o OH CH3 C CH3 H isopropyl alcohol B.p = 106o 3,5-dinitrobenzoate 85o CH3 CH CH2OH CH3 isobutyl alcohol B.p. = 160o 3,5-dinitrobenzoate: 108-110o H OH cyclohexanol B.p. = 155-157o 2,4-DNP = 158 160o O cyclohexanone B.p. = 180 -183o Benzenesulfonamide 110 - 112o Benzamide 160 - 163o NH2 aniline