Organic Chemistry

Organic Chemistry
Introduction
Important definitions
HOMOLOGOUS SERIES – a family of organic compounds which all fit the same general formula, neighbouring members differ from each other by
CH
2
, have similar chemical properties and show trends in physical properties.
e.g.
the alkanes all fit the general formula C n
H
2n+2
, members of the family include methane (CH
4
), ethane (C
2
H
6
) and propane (C
3
H
8
).
EMPIRICAL FORMULA – the simplest ratio of moles of atoms of each element in a compound.
MOLECULAR FORMULA – the actual number of moles of atoms of each element in a compound.
STRUCTURAL FORMULA (aka displayed formula or graphical formula) – shows all of the atoms and the bonds between them. e.g. pentane (C
5
H
12
).
H
H
C
H
H
C
H
H
C
H
H
C
H
H
C
H
H
A condensed structural formula can also be used which omits the bonds. So for pentane we can use:
CH
3
CH
2
CH
2
CH
2
CH
3 or
CH
3
(CH
2
)
3
CH
3
The formulas in the data book are called SKELETAL formulas. These must NOT be used in the exam.
STRUCTURAL ISOMERS – compounds with the same molecular formula but a different arrangement of atoms. e.g. C
4
H
10 represents
H
H
C
H
H
C
H
H
C
H
H
C
H
H
butane and
H
H
H
H C
C H
H
C C
H H
H
H
2-methylpropane
FUNCTIONAL GROUP – atom or group of atoms which gives an organic compound its characteristic chemical properties.
C H
For example
CH
3
CH
3
Alkanes
suffix – ane
ethane
C C Alkenes
suffix – ene
For example
CH
2
=CH
2 ethene
C X Halogenoalkanes
X = Cl, Br or I
prefix halo -
For example
CH
3
CH
2
Cl chloroethane
C OH Alcohols suffix – ol or prefix hydroxy-
For example
CH
3
CH
2
OH ethanol
CH
3
CH(OH)COOH 2-hydroxypropanoic acid
C
O
H
Aldehydes
suffix – al
For example
CH
3
CHO ethanal
C O Ketones
suffix –one or prefix oxo-
For example
CH
3
COCH
3
CH
3
COCH
2
COOH propanone
3 – oxobutanoic acid
C
O
OH
Carboxylic acid
suffix – oic acid
For example
CH
3
COOH ethanoic acid
C NH
2
Amines
suffix – amine or prefix amino -
For example
CH
3
NH
2 methylamine
H
2
NCH
2
COOH aminoethanoic acid
C
O
OR
Esters
suffix – oate
For example
CH
3
COOC
2
H
5 ethyl ethanoate
Aromatic compounds
Contain the
BENZENE ring
H
H
H
C
C
C
C C
C
H
H
H formula C
6
H
6
Delocalised electrons
Some examples
nitro group
CH
3
CHCH
2
CH
3
NO
2
(1-methylpropyl) benzene nitrobenzene
COOH
1
Br
1
2
Cl
1-bromo-2-chlorobenzene
3 CH
3
4
Cl
4-chloro-3methylbenzenecarboxylic acid
Sometimes the benzene ring is not regarded as the key part of the molecule.
In these cases it is referred to as the
PHENYL group.
For example
NH
2 phenylamine
O
CH
3
CH
2
CH
2
C
H
O
CH
3
CH
2
CCH
2
CH
3 aldehyde butan al ketone pentan -3-one
CH
3
CH
3
CHCH
2
C
O
OH carboxylic acid
3-methylbutan oic acid
alcohol H O ketone
O
C alkene
CH
C
C
CH
O ester
O
C
O
H O carboxylic acid
carboxylic acid
O
C
H
2
C ester
OH
O
CH ether
O
CH
C
O aldehyde
CH
2
C
O
H NH
2 amine
H
2
C
C
N nitrile
Physical Properties of Organic
Molecules
Alkanes worksheet
What type of intermolecular force would you expect to find between alkanes, halogenoalkanes, aldehydes, ketones, alcohols and carboxylic acids?
Use this information to deduce the relative boiling points of these homologous series and their solubility in water.
The Alkanes
The alkanes is an homologous series where all members fit the general formula C n
H
2n+2
.
They have trends in physical properties e.g. density and m.p. and b.p. all increase with M r
.
They all undergo similar chemical reactions.
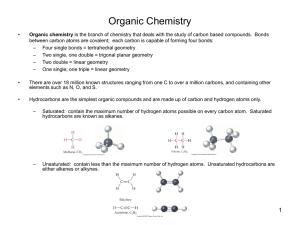
Alkanes are SATURATED HYDROCARBONS. i.e. they contain only single C to C bonds and are made up of C and H atoms only.
Alkanes are obtained from crude oil by fractional distillation.
They are mainly used as fuels.
The large M r alkanes do not ignite easily so there is little demand for them as fuels so they are CRACKED to make smaller more useful alkanes and alkenes.
Apart from combustion alkanes undergo few chemical reactions. This is for two main reasons:
1. The bonds in alkanes are relatively strong.
2. The bonds have a relatively low polarity as the electronegativity of C and H is similar.
As a consequence alkanes can be used as lubricating oils, although they do degrade over time.
Reactions of Alkanes:
Combustion:
Alkanes burn exothermically to produce carbon dioxide and water if there is a plentiful supply of oxygen. This is known as complete combustion.
e.g. CH
4
+ 2O
2
CO
2
+ 2H
2
O
Write equations for the complete combustion of butane and octane.
If there is a limited supply of oxygen incomplete combustion occurs and carbon monoxide or carbon are formed instead of carbon dioxide.
e.g. CH
4
CH
4
+ 1 ½O
2
+ O
2
CO + 2H
2
O
C + 2H
2
O
What problems do the gases released on combustion of alkanes cause?
Chlorination
Methane does not react with chlorine in the dark but in the presence of ultraviolet light reacts to form hydrogen chloride.
CH
4
+ Cl
2
CH
3
Cl + HCl
The mechanism for this reaction is known as free radical substitution.
Substitution = replacement of an atom or group of atoms by a different atom or group of atoms.
Free radical = species with an unpaired electron.
Free radicals are formed by homolytic fission of bonds.
In homolytic fission one electron from the shared pair goes to each atom.
So
Cl Cl
Cl + Cl or
Cl
2
2Cl
.
unpaired electron
Heterolytic fission of Cl – Cl would result in the formation of Cl + and Cl .
There are three steps in the mechanism: initiation, propagation and termination
Free radical substitution chlorination of methane i.e. homolytic breaking of covalent bonds
Overall reaction equation
CH
4
+ Cl
2
CH
3
Cl + HCl
Conditions ultra violet light (breaks weakest bond) excess methane to reduce further substitution
Free radical substitution mechanism
UV Light
Cl
2
Cl + Cl initiation step
CH
4
+ Cl
CH
3
+ Cl
2
CH
3
+ HCl
CH
3
Cl + Cl two propagation steps
CH
3
+ Cl CH
3
Cl termination step
CH
3
+ CH
3
CH
3
CH
3 minor termination step
Also get reverse of initiation step occurring as a termination step.
Further free radical substitutions
Overall reaction equations
CH
3
Cl + Cl
2
CH
2
Cl
2
+ HCl
CH
2
Cl
2
+ Cl
2
CHCl
3
+ HCl
CHCl
3
+ Cl
2
Conditions ultra-violet light excess chlorine
CCl
4
+ HCl
Write down two propagation steps to explain the formation of dichloromethane.
Methane reacts in exactly the same way with bromine to form hydrogen bromide together with bromomethane, dibromomethane, tribromomethane and tetrabromomethane.
Write down the mechanism for the reaction between chlorine and ethane to form chloroethane.
Use the mechanism to explain why small amounts of butane are formed. How could the formation of further substitution products be minimised?
The Alkenes
All fit the general formula C n
H
2n
.
Are unsaturated hydrocarbons as they contain a C = C.
Much more reactive than alkanes.
Industrial importance of alkenes:
1.
Making polymers (plastics)
2.
Hydrogenation of vegetable oils to make margarine
3.
Hydration of ethene to make ethanol.
When naming alkenes have to include position of double bond, for example:
CH
3
CH=CHCH
3 is but - 2 - ene and
CH
3
CH
2
CH=CH
2 is but -1- ene
Draw out and name all of the alkenes with the molecular formula C
6
H
12
.
Alkenes undergo ADDITION reactions.
Two substances combine to form one new substance. Unsaturated molecules are converted to saturated molecules.
Reactions of Alkenes
1. Addition of hydrogen (hydrogenation)
Alkenes react with hydrogen in the presence of a nickel catalyst at 180 °C to form an alkane. e.g.
H H
H H
C = C + H
2
H – C – C – H
H H
H H
C
2
H
4 ethene
+ H
2
C
2
H
6 ethane
Most oils are esters of propane-1,2,3-triol
(aka glycerol) with 3 long chain carboxylic acids (aka fatty acids).
The esters are sometimes called triglycerides.
Hydrogenation of these oils produces margarine.
The common fatty acids include
• octadeca-9-enoic (oleic) acid – unsaturated acid found in most fats and olive oil
• octadeca-9,12-dienoic (linoleic) acid – unsaturated acid found in many vegetable oils such as soyabean and corn oil
CH
2
OOC(CH
2
)
7
CH=CH(CH
2
)
7
CH
3
CHOOC(CH
2
)
7
CH=CH(CH
2
)
7
CH
3
CH
2
OOC(CH
2
)
7
CH=CH(CH
2
)
7
CH
3
Above is the triglyceride formed between propan-1,2,3-triol and oleic acid.
Hydrogenation using a nickel catalyst and slight pressure removes some of the C=C.
This enables the chains to pack together more closely which increases the van der
Waals forces and hence m.p. so the oils are solidified forming margarine.
2. Addition of halogens (halogenation)
Halogens react with alkenes at room temperature and pressure in a non-polar solvent to form a dihalogenoalkane.
e.g.
H H
H
C = C
H
+
Br
2
H
H H
– C – C – H
Br Br
C
2
H
4 ethene
+ Br
2
C
2
H
4
Br
2
1,2-dibromoethane
Bromine water is used as a test for unsaturation.
In the presence of an alkene, bromine water turns from red brown to colourless. Alkanes do not react with bromine water.
Bromine Water Test For Alkenes
C = C + Br
2
(aq) colorless amber
– C – C –
Br Br colorless
3. Reaction with hydrogen halides
(hydrohalogenation)
Alkenes react with hydrogen halides
(HCl, HBr etc.) to form halogenoalkanes.
The reaction occurs at room temperature and pressure. e.g.
H H H H
H
C = C + HBr H – C – C – H
H
H Br
C
2
H
4 ethene
+ HBr CH
3
CH
2
Br bromoethane
4. Hydration (reaction with water)
This can be done in two ways: a) Addition of concentrated sulphuric acid to form an alkyl hydrogensulphate.
Water is then added to hydrolyse the product and produce an alcohol.
The sulphuric acid is regenerated.
H H
H
C = C
H
+ H
2
SO
4
H H
H – C – C – H
H OSO
3
H
H
H H
– C – C – H
H OSO
3
H
+ H
2
O
H H
H – C – C – H
H OH
+
H
2
SO
4
C
2
H
4
+ H
2
SO
4
CH
3
CH
2
OSO
3
H
CH
3
CH
2
OSO
3
H + H
2
O C
2
H
5
OH + H
2
SO
4 ethanol
b) Alkenes can also undergo direct hydration to form an alcohol. Ethene can be converted to ethanol by reaction with steam in the presence of a phosphoric(V) acid (H
3
PO
4
) catalyst at a pressure of 60 –
70 atm and a temperature of 300 °C.
H H H H
C = C + H
2
O H – C – C – H
H H
H OH
What advantages and disadvantages does this method have over production of ethanol by fermentation?
5. Addition Polymerisation
The formation of polymers involves alkenes reacting with themselves to form a long chain molecule called a polymer.
The individual molecules used to make the polymer are called monomers.
Ethene is polymerised to form poly(ethene) n CH
2
= CH
2
CH
2
CH
2 n
n = about 100 to
10 000
CH
2
CH
2 is the repeating unit
monomer repeating unit
CH
2
=CH
2 polymer
- CH
2
– CH
2
poly(ethene) polythene typical uses film, bags
CH
2
=CHCH
3
- CH
2
– CH -
CH
3 poly(propene) polypropylene
Moulded plastic, fibres
CH
2
=CHC
6
H
5
- CH
2
– CH poly(phenylethene) polystyrene packaging, insulation
C
6
H
5
monomer repeating
CF
2
=CF
2 unit
CH
2
=CHCl - CH
2
– CH -
Cl
- CF
2
– CF
2
polymer poly(chloroethene) polyvinylchloride typical uses pipes, flooring
PVC
Poly
(tetrafluoroethene)
Non-stick coating
(Teflon)
PTFE
Alcohols
Alcohols are the homologous series with the general formula C n
H
2n+1
OH.
They all contain the functional group,
OH, which is called the hydroxyl group.
Alcohols can be classified as primary, secondary or tertiary, depending on the carbon skeleton to which the hydroxyl group is attached.
RCH
2
OH
1 alkyl group on C next to OH so primary alcohol, 1 °
R
2
CHOH
2 alkyl groups on C next to OH so secondary alcohol, 2 °
R
3
COH
3 alkyl groups on C next to OH so tertiary alcohol, 3 °
Draw out the structure, name and classify all the alcohols with the formula C
4
H
9
OH.
H
H
C
H
H
C
H
H
C
H
H
C
H
OH
Butan-1-ol primary
H
H
C
H
H
C
H
H
C
H
C
OH
H
H
Butan-2-ol secondary
H
H
C
H
H
C
H
C
CH
3
H
OH
2-methylpropan-1-ol primary
H
H
C
H
OH H
C C
CH
3
H
H
2-methylpropan-2-ol tertiary
Reactions of Alcohols
1. Combustion
In countries such as Brazil, ethanol is mixed with petrol and used to power cars. Ethanol is less efficient as a fuel than petrol as it is already partially oxidised but does make the country less reliant on supply of petrol.
As it can be produced by fermentation of sugar beet, many consider ethanol a carbon neutral fuel.
2. Oxidation of Alcohols
Primary alcohols are oxidised first to aldehydes, such as ethanal. A suitable oxidising agent is acidified potassium dichromate(VI)
H
H
C
H
C
H H ethanol
OH
Cr
2
O
7
/H +
H
H
C C
H ethanal
H
O
+ H
2
O
An aldehyde still has one hydrogen atom attached to the carbonyl carbon, so it can be oxidised one step further to a carboxylic acid.
H
H
O
C C
H ethanal
H
Cr
2
O
7
/H +
H
H
O
C C
H
OH ethanoic acid
In practice, a primary alcohol such as ethanol is dripped into a warm solution of acidified potassium dichromate(VI).
The aldehyde, ethanal, is formed and immediately distils off, thereby preventing further oxidation to ethanoic acid, because the boiling point of ethanal
(23 ° C) is much lower than that of either the original alcohol ethanol (78 ° C) or of ethanoic acid
(118 ° C). Both the alcohol and the acid have higher boiling points because of hydrogen bonding.
If oxidation of ethanol to ethanoic acid is required, the reagents must be heated together under reflux to prevent escape of the aldehyde before it can be oxidised further.
Secondary alcohols are oxidised to ketones. These have no hydrogen atoms attached to the carbonyl carbon and so cannot easily be oxidised further.
H
H
C
H
C
H
C
H OH H propan-2-ol
H
Cr
2
O
7
/H +
H
H
C C
H
C
H O H propanone
H
Distinguishing between 1 °, 2° and 3° alcohols
When orange acidified potassium dichromate(VI) acts as an oxidising agent, it is reduced to green chromium(III) ions.
Primary and secondary alcohols both turn acidified dichromate(VI) solution from orange to green when they are oxidised, and this colour change can be used to distinguish them from tertiary alcohols.
Tertiary alcohols are not oxidised by acidified dichromate(VI) ions, so they have no effect on its colour, which remains orange .
Halogenoalkanes
Named by using the name of the alkane from which they are derived with the prefix chloro-
, bromo- or iodo-.
For example:
CH
3
CH
2
Br is bromoethane
(CH
3
)
2
CHCH
2
Cl is 1-chloro-2-methylpropane
Remember the position of the halogen atom must be indicated using the appropriate number so
CH
3
CH
2
CH
2
Cl is 1-chloropropane and
CH
3
CHClCH
3 is 2-chloropropane
Halogenoalkanes can be classified in the same way as alcohols.
Draw out, name and classify all the isomers with the formula C
5
H
11
Br.
Key feature of halogenoalkanes is
C X where X = Cl, Br or I
What is notable about this bond compared with say, C – C and C – H?
The halogen atom is more electronegative than C so the bond is polarised:
+
-
C X
ORDER OF BOND
POLARITIES:
+
-
C Cl >
+
-
C Br >
+
-
C I
So is order of reactivity: chloroalkane > bromoalkanes > iodoalkanes?
Is there another factor that ought to be considered before reaching a conclusion?
BOND
ENERGIES
Bond energies:
Bond
C - Cl
C - Br
C - I
Bond energy in kJmol -1
346
290
234
This suggests that the order of reactivity is: iodoalkane > bromoalkanes > chloroalkanes
There’s only one way to find out which is best!
Do an experiment, not fight!
But what do halogenoalkanes react with?
The
+ carbon atom is susceptible to attack by
NUCLEOPHILES.
A nucleophile is a species with a lone pair of electrons.
e.g. OH , NH
3
, CN .
When attack by a nucleophile occurs, the carbon – halogen bond breaks releasing a halide ion.
A suitable nucleophile for experimentation is OH from an aqueous solution of an alkali such as sodium hydroxide.
CH
3
CH
2
X + OH CH
3
CH
2
OH + X -
X = Cl, Br or I.
OH has replaced the X so overall we have
NUCLEOPHILIC SUBSTITUTION
How can we follow the rate of this reaction so that we can determine the order of reactivity?
Halide ions coloured precipitates when silver nitrate is added.
So we can measure how long it takes for a precipitate to form.
See EXPERIMENT SHEET.
Mechanisms for nucleophilic substitution
S
N
1 = unimolecular nucleophilic substitution
(only one species in the slow step of the mechanism, rate determining step)
S
N
2 = bimolecular nucleophilic substitution
(two species in the slow step of the mechanism, rate determining step)
Use of curly arrows:
Curly arrows are used in reaction mechanisms to show the movement of electron pairs.
TAILS come from either a bond pair of electrons
HEADS point either next to an atom to form a bond pair of electrons
X or a lone pair of electrons or at an atom to form a lone pair of electrons
X
Heterolytic fission of
C – Br bond
H
CH
3
C
H
Br
CH
3
H
C +
H
H
CH
3
C + +
H
Intermediate carbocation
H
CH
3
C
H
OH
Br
slow fast
S
N
1
OH
-
H
OH
-
CH
3
C
H
Br
HO
H
C
CH
3
H
Br
-
Transitio n state
HO
H
C
H
CH
3
+ Br
-
S
N
2
Which is best? S
N
1 or S
N
2?
For primary halogenoalkanes – S
N
2
For tertiary halogenoalkanes – S
N
1
3 ° halogenoalkanes cannot undergo the S
N
2 mechanism as 5 bulky groups would not fit around the
C in the transition state - steric hindrance.
1 ° halogenoalknes are less likely to undergo S
N
1 as this would involve the formation of a primary carbocation as an intermediate.
Alkyl groups push electron density to the C atom they are attached to
(positive inductive effect) which stabilises the positive charge.
More alkyl groups mean a more stable carbocation.
2 ° halogenoalkanes react via a mixture of S
N
1 and S
N
2.
The mechanism predominating depends upon the nature of the alkyl groups and the nature of the solvent.