05_physical properties
advertisement
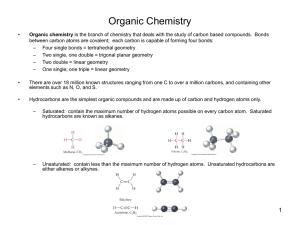
Organic Chemistry Physical Properties Physical Properties If the functional groups are the same, the length of the carbon chain tells us which organic compound will have higher melting and boiling points. Longer carbon chain means higher melting and boiling points Reason: increasing the length of the non-polar chain increases the molar mass of the chain, which increases the strength of the dispersion forces between the molecules. Example: hexan-1-ol has higher melting and boiling points than ethanol Name Structural formula Melting point (°C) Boiling point (°C) ethanol HO – CH2 – CH3 -114 78.4 hexan-1-ol HO – CH2 – CH2 – CH2 – CH2 – CH2 – CH3 -46.7 158 Physical Properties If the lengths of the carbon chain are similar, the polarity of the functional groups tells us which organic compound will have higher melting and boiling points. More polar groups means higher melting and boiling points Reason: more polar groups means stronger dipole-dipole forces between molecules Example: pentan-1-ol has higher melting and boiling points than pentanal Explanation: the O-H bond is more polar than the C=O bond. The pentan-1-ol will display hydrogen bonding between its molecules, pentanal will not. Name Structural formula Melting point (°C) Boiling point (°C) O pentanal pentan-1-ol H – C – CH2 – CH2 – CH2 – CH3 HO – CH2 – CH2 – CH2 – CH2 – CH3 -60.0 102 -77.6 138 Physical Properties An isomeric acid of an ester is a carboxylic acid that has the same number of each element as the ester. (They have the same molecular formula.) Esters have lower boiling points than isomeric acids. Reason: The ester and its isomeric acid have the same length of non-polar carbon chain, so the boiling point depends on the polarity of the groups. Carboxylic acids contain a highly polar O-H bond so the molecules will display hydrogen bonding. The secondary interactions in the ester will be weaker. Example: pentanoic acid has a higher boiling point than ethyl propanoate (both have molecular formula C5H10O2) Name Structural formula O ethyl propanoate CH3 – CH2 – O – C – CH2 – CH3 pentanoic acid O HO – C – CH2 – CH2 – CH2 – CH3 Boiling point (°C) 99.1 186 Physical Properties In general, compounds with similar polarity dissolve each other (like dissolves like). Water is highly polar and displays hydrogen bonding between its molecules. Compounds with more polar groups and lower molar mass will be more soluble in water. Example: ethanol is more soluble in water than propanol (ethanol has a lower molar mass) Example: ethanol is more soluble in water than ethanal (a hydroxyl group is more polar than an aldehyde group) Example: butan-2,3-diol is more soluble than butan-2-ol (butan-2,3-diol has two hydroxyl groups, butan-2-ol only has one)