PowerPoint 2007
advertisement

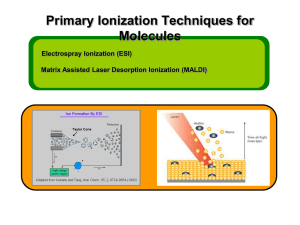
Analyzing Biological and Organic Polymers by MALDI-TOF Jonathan A. Karty, Ph.D. Topics Covered Sample Requirements Instrument Overview General Instrument Use Instructions Tips and Tricks What is the Bruker Autoflex III? Time-of-flight mass spectrometer Ions of given same kinetic energy, heavy ions travel slower than lighter ones Two modes of operation Linear Reflectron MALDI/LDI source 384 position target plate (~1 µL spot size) 355 nm Nd:YAG laser Can analyze positive or negative ions (same spot) Autoflex III Picture Matrix-Assisted Laser Desorption/Ionization (MALDI) Analyte is mixed with UV-absorbing matrix A drop of this liquid is dried on a target Analyte incorporated into matrix crystals Spot is irradiated by a laser pulse ~10,000:1 matrix:analyte ratio Analyte does not need to absorb laser Irradiated region sublimes, taking analyte with it Matrix is often promoted to the excited state Charges exchange between matrix and analyte in the plume (very fast <100 nsec) Ions are accelerated toward the detector MALDI Diagram Image from http://www.noble.org/Plantbio/MS/iontech.maldi.html MALDI Advantages Technique is relatively simple Volatilize and ionize labile molecules Imagine electron ionization on a protein MALDI creates very simple mass spectra Ions are usually (M+nH)n+ or (M-nH)nOnly 1-3 charge states are observed MALDI ideal for time-of-flight analyzers Usually 1 charge state for peptides < 3.5 kDa Theoretically unlimited mass range (100 kDa done here) MALDI is very rapid (<1 min/spot) Low sample consumption (1 µL) Wide array of matrices available for different analytes Some Common MALDI Matrices What Samples Can It Run? Biopolymers Organometallic complexes Organometallic salts work great Synthetic polymers Peptides, proteins, DNA, RNA, oligosaccharides Polymer need not be soluble in same solvent as matrix Molecules that photoionize upon irradiation by 355 nm laser Porphyrins Organometallic complexes What Samples Can’t It Run? “Dirty” samples Significant concentration of involatiles Alkali metal salts can be quite problematic RNA/DNA analyses require extensive desalting Molecules with significant vapor pressures Glycerol, urea, most buffers, many detergents Instrument is held at ~10-7 torr Molecules that do not make stable ions in source Lack charge acceptor/donor site Cannot photoionize with Nd:YAG laser MALDI Advantages Relatively gentle ionization technique Very high MW species can be ionized Molecule need not be volatile Very easy to get sub-picomole sensitivity Spectra are easy to interpret Positive or negative ions from same spot Wide array of matrices available MALDI Disadvantages MALDI matrix cluster ions can obscure low m/z (<600) range Analyte must have very low vapor pressure Coupling MALDI with chromatography can be difficult Analytes that absorb the laser can be problematic Fluorescein-labeled peptides Instrument Diagram 355 nm Nd:YAG laser Target Reflectron Linear Detector Lens Extraction Plate Flight Tube Entrance Reflector Detector Linear Mode 355 nm Nd:YAG laser Target Reflectron Linear Detector Lens Extraction Plate Flight Tube Entrance Reflector Detector Linear mode is used for large (>3.5 kDa) molecules or exceedingly fragile species (oligosaccharides). It is capable of 4,000 resolving power @ 3.2 kDa (1,000 RP @ 12 kDa) Reflectron Mode 355 nm Nd:YAG laser Target Reflectron Linear Detector Lens Extraction Plate Flight Tube Entrance Reflector Detector Reflectron mode is used for small species (<4 kDa) and is capable of 11,000 resolving power @ 3.2 kDa. (ACTH 7-38+H)+ (Ubiq+2H)2+ (ACTH 18-37+H)+ MALDI Example (Ins+H)+ (Ubiq+H)+ MALDI Example I Continued Intens. [a.u.] Intens. [a.u.] MALDI-TOF Example 2 8476.61 16952.53 4000 6000 3000 16952.53 4000 2000 1000 2000 17056.51 17131.54 0 4802.98 16600 16800 17000 17200 1740 0 5000 7500 10000 12500 15000 17500 20000 22500 25000 27500 m/z General Sample Guidelines Purify analyte if possible Use only volatile solvents/buffers MeOH, H2O, acetone, CH3CN, THF, CH2Cl2, C6H6 TFA, HOAc, formic acid, NH3, etc. Ionic strength < 20 mM (e.g. 0.1% v/v HOAc) If you need a detergent, 20 mM noctylglucopyranoside can work Analyte should be 5 – 100 µM in concentration ZipTips can help purify dirty samples (C4 and C18 available in MSF) No SDS, TWEEN, CHAPS, etc Need at least 2 µL Sample Prep Tricks Ziptip to clean up dirty samples C18 for peptides < 3 kDa C4 for peptides/proteins > 3kDa Elute directly into matrix for added sensitivity ZipTip instructions on MSF website If CCA liquid turns yellow, pH is too high Spots from non-acidic CCA do not crystallize correctly Add a little 1% v/v or 10% v/v TFA to lower pH If sample needs base for solubility, try over-layer method Dissolve sample in NH3 or other volatile base Place 1 uL of sample on target, let dry completely Deposit 1 uL matrix over top of dried sample Sample Prep Tricks 2 Non-aqueous over-layer Make 1 uL spot of matrix on plate, let dry Deposit small amount of sample in volatile solvent (e.g. CHCl3, acetone, CH2Cl2) You can even do internal calibration this way Put calibrants in matrix spot For better mass accuracy, let voltages stabilize 10-30 minutes before recording data Hands-on Training Starts AFTER 11/7 Groups of no more than three One hour or so to complete No charge for first session After training, students must demonstrate competency by running their own samples prior to being granted after-hours access