Stationary phase
advertisement

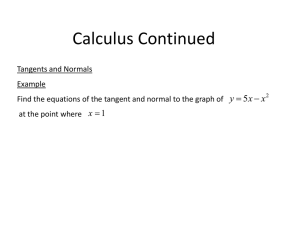
Advanced Fractionation Techniques for Complex Polyolefins Harald Pasch SASOL Chair of Analytical Polymer Science Department of Chemistry and Polymer Science, University of Stellenbosch, South Africa 1 Polyolefin Analysis Complex Structures New Copolymers Cl Zr + R R = n-B uty l, n-D ecyl or n-H ex adecy l Fractionation Techniques ? 1 Cl MAO New Applications fraction R chemical Chemical composition composition TREF CRYSTAF 2 Molecular hydrodynamic size volume HT-SEC Polyolefins – The Most Common Polymers H2 C H2 C C H2 H2 C H2 C C H2 C H2 n polyethylene CH CH 3 CH 3 CH 3 3 CH H 3C C H2 C H2 n C H2 polypropylene 3 C H2 3 Where are the problems ? Polyolefins just CH, CH2 and CH3 .... ????? H 3C H 3C H 3C H 3C H 3C H 3C H 3C H 3C H 3C Isotactic polypropylene (high crystallinity) Narrower PDI (Metallocene) Syndiotactic polypropylene Atactic polypropylene (low crystallinity) 4 Broader PDI (Ziegler-Natta) Polyolefins just CH, CH2 and CH3 .... ????? 1 2 3 Molecular structure Morphology Physical Properties MM,MMD SCB distribution SCB content Crystal size, crystal size distribution tie molecules interfacial order haze,gloss, clarity, tear strength, tensile strength,impact strength Instrumentation required Instrumentation required Instrumentation required HT-SEC CRYSTAF, TREF DSC NMR, FTIR DSC, DMA NMR, Microscopy AFM, SEM Different test equipment 5 Why does chain structure influence properties? Structures LDPE Density 0.935 6 LLDPE 0.929 -0.945 HDPE 0.940 - 0.965 Branch type influences the crystal structure Distribution of the branches ??? Change in SEM Crystal Morphology as a Result of Blending Results: LDPE 60% LDPE + 40% Plastomer poly(ethylene-1-octene) 7 Molar Mass Analysis 8 High-Temperature SEC Polymer Labs Model PL GPC 220 Stationary phase: Cross-linked PS Mobile phase: Trichlorobenzene Temperature: 140 oC Calibration: PS, PE Detectors: RI, ELSD, IR, LS, Vis 9 HT-SEC and FTIR of an Oxidized Polyethylene ? 10 Universal LC-FTIR Coupling pump + injector RI-detector Separation HPLC / GPC LCTransform Abs Identification 0.3 5 0.3 0 0.2 5 3. 0 2. 5 0.1 5 0.1 0 2. 0 0.0 5 0.0 0 1. 5 2 000 c m-1 FTIR spectrometer 11 series of spectra Ni c 0.2 0 SEC-FTIR of an Ethylene-Methacrylic Acid Copolymer Gram Schmidt 0.12 -1 1165 ratio 1740 cm /CHn 0.3 90 1377 17/0 3, GK 0 033/58 /2 95 1739 1705 100 -1 80 -1 0.2 n 0.08 718 70 %T ratio 1720 cm /CH 1472 75 0.10 729 ratio 1704 cm /CHn 85 65 50 ratio 55 0.1 0.06 17 /03, G K 003 3/58 /2 9 9.5 9 9.0 9 8.5 45 9 8.0 3500 0.0 3000 9 6.5 2500 2000 1500 Wavenumbers (cm-1) 3500 3000 2500 2000 1500 %Transmi ssion 4000 9 7.0 2915 35 0.04 9 7.5 2848 40 Intensity 60 9 6.0 1000 9 5.5 0.02 1000 9 5.0 9 4.5 9 4.0 -0.1 9 3.5 9 3.0 1705 Wellenzahl [cm-1] 9 2.5 0 10 20 9 1.5 1 850 1 800 30 0.00 1739 9 2.0 1 750 1 700 1 650 1 600 W elle nza hlen (c m -1) Elution volume [ ml ] 1800 12 1750 1700 1650 1600 SEC-FTIR Analysis of a Polyolefin Blend 13 Separation by Crystallizability: Chemical Heterogeneity fraction CRYSTAF chemical composition TREF • • hydrodynamic volume HT-SEC Temperature Rising Elution Fractionation (TREF) Crystallization Analysis Fractionation (CRYSTAF) separation with regard to chemical composition 14 Separation by Crystallizability: Chemical Heterogeneity • Based on Flory-Huggins expression for polymerdiluent mixtures • diluent: solvent, comonomer • melting point depression is a function of noncrystallizable comonomer content • 15 chemical composition separation = separation by crystallizability 1/Tm - 1/Tmo = -(R/DHu) ln NA Tm melting point copolymer Tmo melting point homopolymer DHu heat of fusion per repeat unit NA mole fraction of comonomer Temperature Rising Elution Fractionation D issolve the sa m p le in hot T C B or o-D C B L oa d the colu m n w ith the h ot solutio n C ry stallize the sa m p le by slow tem p.-prog ra m m ed cooling 2 -4 K /hour ta ke 6 0 -3 0 hours E lute th e sa m p le by slow tem p.-prog ra m m ed heating 8 K /hou r ta ke 1 5 -20 hours 16 L . W ild , A d v. P olym . S ci. 9 8 (1 99 0 ) 1 -47 TREF Separation Mechanism The slow cooling rate is the most important factor in achieving good separation The slow cooling rate minimizes the effects of co-crystallization and molar mass influences. Typical cooling rates would be about 2°C/hour It takes about 2-3 days to cool!! 17 Temperature Rising Elution Fractionation Comparison of typical LLDPE and LDPE 18 Temperature Rising Elution Fractionation Hypothetical samples with same MMD and crystallinity distribution but different dependency on each distribution 19 Temperature Rising Elution Fractionation Automatic Cross-Fractionation System TREF-SEC S. Nakano, Y. Goto, J. Appl. Polym. Sci. 26 (1981) 4217 20 Temperature Rising Elution Fractionation TREF-SEC Analysis of a Blend of Two Polyethylenes S. Nakano, Y. Goto, J. Appl. Polym. Sci. 26 (1981) 4217 21 Crystallization Analysis Fractionation IR detector dW/dT W [%] 100 6 4 60 40 2 20 0 20 22 30 40 50 60 70 Temperature [°C] 80 0 90 W [%] dW/dT 80 Crystallization Analysis Fractionation 23 Crystallization Analysis Fractionation 2 140 120 Typical temperature cycle 100 1 4 6 80 5 60 40 Zone 3 Zone 1 Zone 2 16 100 0 100 200 300 400 500 600 700 T im e (m in ) 80 F irs t D e riv a tiv e 12 10 60 8 6 40 S o lu b le fra ctio n 4 20 2 0 0 0 24 10 20 30 40 50 o T e m p e ra tu re ( C ) 60 70 80 d w /d T Typical crystallization curve 14 In fra re d S ig n a l C u m u la tiv e F ra ctio n (% ) o T e m p e ra tu re ( C ) 3 Crystallization Analysis Fractionation Crystaf analysis of a PP blend Comparison of TREF and CRYSTAF 25 CRYSTAF: Analysis of Copolymers and Blends 35 HDPE 60 15 40 10 0 0 30 40 50 60 70 80 4 2 20 5 LLDPE 6 80 20 20 Lupolex 25 Q F A 100 W [% ] d W /d T 25 Lupolex 18 K F A 8 d W /d T 30 W dW /dT 0 20 90 30 40 60 70 80 90 100 T em perature [°C ] T em perature [°C ] W d W /d T 12 50 HDPE/PP 50 : 50 d W /d T 8 HDPE/LDPE 4:96 100 100 10 80 40 2 20 0 0 30 26 40 50 60 70 80 T e m p e ra tu re [°C ] 90 100 d W /d T 4 60 4 40 2 0 30 20 0 40 50 60 70 80 T e m p e ra tu re [°C ] 90 100 W [% ] 60 6 80 6 W [% ] d W /d T 8 CRYSTAF: Analysis of Copolymers and Blends Propene--Olefin Copolymers m elting tem perature (D S C ) cry stallization tem perature (D S C ) cry stallization tem perature (C R Y S T A F ) 160 15 Propene-Octene 8.4 8.6 8.8 o 8.2 10 T em perature [ C ] 140 dW /dT 3.1 5 120 100 80 60 40 20 0 40 50 60 70 80 90 100 0 0 o T em p eratu re [ C ] 1 2 16 com on om er [m ol-% ] 18.10 14 12 18.9 18.4 18.11 3 Propene-Octadecene Propene-Olefin Copolymers 18.6 10 Crystallization Temperature dw /dT 3.1 8 6 4 2 0 40 50 60 70 80 90 100 80 75 70 65 Octene 60 55 50 Decene 45 40 PP Tetradecene Octadecene 35 30 0 1 2 o T em p eratu re [ C ] 27 % Comonomer 3 4 CRYSTAF (in particular when coupled to IR sensor) excellent technique but very time consuming Fast and selective techniques are required liquid chromatography is a good candidate Polyolefins are soluble only at high temperatures unconventional stationary and mobile phases ??? 28 Elution Behaviour of Polyolefins in High Temperature Chromatography (HT-HPLC) Using Interactive Stationary Phases polyolefin must dissolve in the mobile phase screening of solubility polyolefin must interact with the phase system screening of mobile and stationary phases 29 Solvents and Columns Trichlorobenzene Cyclohexanone polarity Decaline Dimethylformamide normal phase systems: SiO2 (ZrO2, TiO2, Al2O3) reversed phase systems: Diol… CN… Phenyl… polarity 30 C8… C18 Screening of Stationary Phases for HT-HPLC lo g M Stationary phase: 5 dimethylsiloxanemodified silica gel CH3 4 Cl Si Cl CH3 Benzylalcohol CH3 CH3 H 2O OH SiO 2 Si 3 O x Cyclohexylacetate O n OH DMF 2 SiO 2 Cyclohexanone 31 3 e lu tio n tim e [m in ] 4 Screening of Stationary Phases for HT-HPLC Stationary phase: dimethylsiloxane-modified silica gel, solvent: TCB lo g M SEC conditions with regard to PP () Limiting conditions with regard to PE () 100000 10000 1000 mobile phase: EGMBE 2 .0 32 2 .5 3 .0 3 .5 e lu tio n tim e [m in ] 4 .0 Screening of Stationary Phases for HT-HPLC Stationary phase: dimethylsiloxane-modified silica gel, solvent: TCB PE PP: SEC PE: Limiting conditions PP 36k + PE 34k [V ] 0 .2 P P 3 6 kg /m o l 0 .1 P P 5 7 kg /m o l P P 4 3 8 kg /m o l PP 57k + PE 66k PP 438k + PE 500k 0 .0 1 .5 mobile phase: EGMBE 33 2 .0 2 .5 3 .0 3 .5 e lu tio n tim e [m in ] 4 .0 4 .5 Analysis of PE-PMMA Block Copolymers: SEC and FTIR 0 .3 5 1 .0 PMMA ? PE 9 9 3 .3 1 0 .1 0 0 .3 7 1 7 .5 2 0 .1 5 0 .4 7 5 8 .7 8 0 .2 0 1 1 9 1 .5 4 0 .5 A bsorbance 0 .6 0 .2 0 .0 5 9 5 5 .2 2 8 4 0 .5 0 0 .2 5 0 .7 1 1 4 8 .3 4 0 .3 0 A bsorbance D e te c to r S ig n a l (m V ) 0 .8 1 8 9 11 1 7 2 8 .9 8 T T T T 0 .9 0 .1 0 .0 0 0 .0 3 1 x1 0 4 1 x1 0 5 1 x1 0 6 1 x1 0 7 1 x1 0 M o la r M a s s (g /m o l) 4000 3000 2000 -1 W ave n u m b ers (cm ) 1000 y Multimodal distribution blend or copolymer ? Chemical composition as a function of molar mass ? 34 MMA and ethylene units can be identified, but is it a copolymer or a polymer blend ? Analysis of PE-PMMA Block Copolymers: Coupled SEC-FTIR 0 ,5 G ra m -S c h m id t A G ra m -S c h m id t -1 PE W a v e h e ig h t C h e m ig ra m (1 7 3 0 c m ) 1 ,0 W a v e h e ig h t C h e m ig ra m (1 7 3 0 c m ) -1 W a v e h e ig h t C h e m ig ra m (7 2 0 c m ) PMMA C -1 -1 W a v e h e ig h t C h e m ig ra m (7 2 0 c m ) E t / (E t + M M A ) 0 ,4 E t / (E t + M M A ) 0 ,8 S ig n a l In te n sity Chemical composition as a function of molar mass is visualized ! 0 ,2 0 ,1 0 ,2 0 ,0 0 ,0 0 5 10 15 R e te n tio n T im e (m in ) PE-b-PMMA 20 25 0 B -1 W a v e h e ig h t C h e m ig ra m (7 2 0 c m ) G ra m -S c h m id t 5 Homopolymers and copolymers can be D identified ! 10 15 20 25 R e te n tio n T im e (m in ) -1 W a v e h e ig h t C h e m ig ra m (1 7 3 0 c m ) E th yle n e R a tio 0 ,4 E th yle n e R a tio S ig n a l In te n sity 0 ,3 0 ,6 G ra m -S c h m id t 0 ,6 -1 W a v e h e ig h t C h e m ig ra m (1 7 3 0 c m ) 0 ,3 -1 W a v e h e ig h t C h e m ig ra m (7 2 0 c m ) E t / (E t + M M A ) E t / (E t + M M A ) 0 ,5 0 ,2 S ig n a l In te n sity 0 ,3 0 ,2 E th yle n e R a tio PE 0 ,1 E th yle n e R a tio S ig n a l In te n sity 0 ,4 0 ,0 0 ,1 0 ,0 -0 ,1 -0 ,1 0 5 10 15 R e te n tio n T im e (m in ) 35 20 25 0 5 10 15 R e te n tio n T im e (m in ) 20 25 Analysis of PE-PMMA Block Copolymers What about interaction chromatography ? SEC molar mass separation LC-CC 36 chemical composition separation Analysis of PE-PMMA Block Copolymers: Gradient HPLC 2 A Column: Nucleosil 300 C18 Temperature: 140C Mobile phase: gradient from100 % DMF to 100 % TCB 1 4 3 1- P M M A M n = 8 2 9 0 0 0 g /m o l 2- P M M A M n = 2 2 2 0 0 g /m o l 3- P M M A M n = 1 7 3 0 g /m o l 4- P E M n = 1 1 1 0 g /m o l 6 5 a + b - P E M n = 1 2 0 0 0 g /m o l 6- P E M n = 1 1 4 0 0 0 g /m o l 5b S ignal Intensity (m V ) 5a B 7 PMMA 8 PE 9 T1 PE-b-PMMA 10 T8 T9 0 2 4 6 8 10 12 o T e m p e ra tu re ( C ) 37 14 16 18 20 22 Analysis of PE-PMMA Block Copolymers: Gradient HPLC-FTIR 10 A 1 G ra m -S c h m id t -1 ---- PMMA 1730 cm P e a k -h e ig h t C h e m ig ra m (1 7 3 0 c m A b so rp tio n In te n sity 8 8x -1 ) -1 P e a k -h e ig h t C h e m ig ra m (7 2 0 c m ) ----- PE 720 cm-1 6 PE 4 2 3 2 0 0 2 4 6 8 10 12 14 16 18 20 22 High-Temperature Gradient HPLC as a New Tool for the Analysis of Olefin Copolymers 24 R e te n tio n T im e (m in ) 10 B 4 5 G ra m -S c h m id t -1 PMMA A b so rp tio n In te n sity 8 P e a k -h e ig h t C h e m ig ra m (1 7 3 0 c m ) 5x -1 P e a k -h e ig h t C h e m ig ra m (7 2 0 c m ) 6 6 PE-b-PMMA 7 4 2 0 0 38 2 4 6 8 10 12 14 16 R e te n tio n T im e (m in ) 18 20 22 24 Polymer Labs‘ High-Temperature Gradient HPLC System 39 Separation System for PE-PP Blends PP PE M signal % TCB Time [min] 40 Separation System for PE-PP Blends 1 ,0 1,5 B le n d M o p le n H P u n d P E 1 2 8 .0 0 0 PE PP propylene-rich Detektos Signal (V) 0 ,8 0 ,6 E L S D [V ] BASELL EPM-S1-D 20837-28 0 ,4 0 ,2 1,0 ethylene-rich PP 0,5 0 ,0 0,0 0 2 4 6 8 10 E lu tio n s v o lu m e n (m l) 12 0 2 4 6 8 10 12 Elutions Volumen (mL) column: Nucleosil 500 mobile phase: EGMBE-TCB EP copolymer with 48% ethylene T: 140oC detector: ELSD sample solvent: TCB 41 L.-C. Heinz, H. Pasch, High-Temperature Gradient HPLC for the Separation of Polyethylene-Polypropylene Blends.Polymer 46 (2005) 12040 Separation System for EVA Copolymers EVA 5% VA EVA 12% VA EVA 14% VA EVA 19% VA EVA 28% VA EVA 45% VA EVA 50% VA EVA 60% VA EVA 70% VA PVAc-St. 164KD PVAc-St. 32KD PE-St. 126KD 10 8 6 4 100 stationary phase: silica gel mobile phase: gradient of decaline-cyclohexanone 80 60 PVAc 40 PE % Cyclohexanon Detektorsignal ELSD [V] 12 20 2 0 0 0 5 10 15 20 Elutionsvolumen [ml] 42 A. Albrecht, R. Brüll, T. Macko, H. Pasch: Separation of Ethylene-Vinyl Acetate Copolymers by High-Temperature Gradient Liquid Chromatography. Macromolecules 40 (2007) 5545 Separation of Polyolefins by Tacticity 1,0 response of ELSD [Volts] 0,8 isotactic PP atactic PP linear PE syndiotactic PP 0,6 0,4 0,2 Start of gradient 0,0 0 5 10 15 20 elution time [minutes] 25 stationary phase: carbon-based mobile phase: gradient of 1-decanol-TCB 43 Schematic Protocol for 2D Separations 1 Vr 2 1 2 44 Two-Dimensional Chromatography (HPLC vs. SEC) 1. Dimension: HPLC/LCCC Degasser Pump Injector 2. Dimension: GPC HPLC Column Data Processing Degasser Detector Pump SEC Column Waste 45 High-Temperature 2D-HPLC in Stellenbosch 46 High-Temperature 2D-HPLC Chromatographic conditions: Stationary phase: Mobile phase: Operating temperature: 47 Hypercarb gradient of decanol-TCB 160 oC Ginsburg, A., Macko, T., Dolle, V., Bruell, R., Europ. Polym. J. 47 (2011) 319-329 High-Temperature LC-NMR 120°C 120°C 120°C 120°C 120°C 120°C waste 48 High-Temperature LC-NMR ELSD Transfer line HT Stop-flow valve Transfer line 49 HT-SEC 1 a) R CH 1 CH 2 R 2 polyethylene n 4 3 b) R CH CH3 R 2 O polymethyl methacrylate n O CH3 2 8 7 c) R CH 7 2 CH 6 2 CH CH3 R 2 O O CH3 5 50 n copolymer EtMMA On-flow High-Temperature SEC-NMR min sec T=130°C 85.0 TCB Impurities 80 80.0 75 75.0 70 70.0 65 65.0 60 60.0 55 55.0 4.0 51 3.5 3.0 2.5 2.0 1.5 1.0 0.5 50 ppm On-flow High-Temperature SEC-NMR Solvent subtraction of impurities min sec T=130°C 85.0 80 80.0 75 PE Mn=1.100 75.0 70 70.0 65 65.0 Et-MMA Mn=10.600 60 60.0 55 flow rate 0.5mL/min, conc. 2+2+2 mg/mL, 300 µL injection volume, 5 Waters columns, 24 scans per FID, 1.24s repetition delay 4.0 52 3.5 3.0 2.5 55.0 2.0 1.5 1.0 0.5 50 ppm PMMA Mn=263.000 On-flow High-Temperature SEC-NMR 1 a) R CH 1 CH 2 R 2 n 1H 4 traces of the on-flow run 3 1 b) R CH CH3 R 2 1O a) R CH 2 O CH R 2 n CH3 -CH3- c) PE 1 n 2 4 7 c)b) R R CH 8 73 7 2 CH3 6 CH CH CH2 2 CH3 R 2 5(m) O O R n n O 6(m) b) Et-MMA 1 6(m) 1 O a) R CH 2 CH3 CH 52 n CH3 4(r) 2(r) 2 3(r) 4 3 b) a) PMMA 4.0 R CH 7 3.5 3.0 2.5 2.0 R 1.5 1.0 c) ppm R CH CH3 R 2 7 2 O CHO 2 n 6 CH 8 CH3 2 O CH3 2 53 O CH3 85 On-flow High-Temperature SEC-NMR Monomer composition (mol%) 100 1,0 80 0,8 E 60 40 0,6 0,4 MMA 20 0,2 0 0,0 10 15 Retention time (min) 4.0 54 3.5 3.0 2.5 2.0 1.5 1.0 0.5 ppm 20 Intensity of NMR projection on-flow HT-SEC-NMR of PE-PMMA Block Copolymer