LABORATORY QUALITY
CONTROL
Course Code RIT 2.2 Revision C
Definitions:
• Quality Control:– the process of detecting errors
• Quality Assurance:– the systems or procedures in place to
avoid errors occurring
… to ensure the reliability of the test
results to give the best patient care !
Unreliable Performance ?
• Potential consequences include:– patient misdiagnosis
– delays in treatment
– increased costs
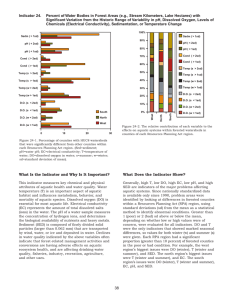
• avoidable retests cost US 200million USD per year
• Even a small calibration bias can effect
treatment rates:
– 1% +ve bias in cholesterol result
5% increase in patients exceeding the treatment cut-off
– 3% +ve bias
15% increase in patient treatment.
Error Classification..
• Pre-analytical:– errors before the sample reaches the laboratory
• Analytical:– errors during the analysis of the sample
• Post-analytical:– errors occurring after the analysis
Pre - Analytical Errors..
• Improper preparation of the patient:– patient fasting
• glucose test
– stress and anxiety
• urinary protein
Pre - Analytical Errors..
• Improper preparation of the patient
• Improper collection of the blood sample:– sample haemolysis
• LDH, potassium or inorganic phosphate
– insufficient sample volume
• unable to carry out all requested tests
– collection timing
• 24 hour urine
Pre - Analytical Errors..
• Improper preparation of the patient
• Improper collection of the blood sample
• Incorrect specimen container:– serum or plasma
– fluoride tubes for glucose
• to inhibit glycolysis
– EDTA unsuitable anti-coagulant for calcium
Pre - Analytical Errors..
•
•
•
•
Improper preparation of the patient
Improper collection of the blood sample
Incorrect specimen container
Incorrect specimen storage:– sample left overnight at room temperature
• falsely elevated K, Pi and red cell enzymes
– delay in sample delivery
• falsely lowered levels of unstable analytes
Other Factors..
• The sex of the patient
– male or female
• The age of the patient
– new born / juvenile / adult / geriatric
• Dietary effects
– low carbohydrate / fat
– high protein / fat
• When the sample was taken
– early morning urine collection pregnancy testing
• Patient posture
– urinary protein in bed-ridden patients
Other Factors..
• Effects of exercise
– creatine kinase / CRP
• Medical history
– heart disease / diabetes / existing medication
• Pregnancy
– hormonal effects
• Effects of drugs and alcohol
– liver enzymes / dehydration
Analytical Errors..
• The sample:
– labelling
• barcoding / aliquoting
– preparation
• centrifugation / aspiration
– storage temperature
• short –term refrigeration
• medium term freezing at –20oC
• long term freezing at -80oC
– correct test selection
• Laboratory Information Management System (LIMS)
Analytical Errors..
• The sample:
• Glassware / pipettes / balances:
–
–
–
–
used incorrectly
contaminated
poorly calibrated
reuse of pipette tips
Analytical Errors..
• The sample:
• Glassware / pipettes / balances:
• Reagents / calibrators / controls:
– poor quality
– inappropriate storage
• correct temperature
• badly maintained fridges or freezers
– stability
• shelf-life / working reagent
– incorrect preparation
Analytical Errors..
•
•
•
•
The sample:
Glassware / pipettes / balances:
Reagents / calibrators / controls:
The application:
– incorrect analytical procedures
– poorly optimised instrument settings
Analytical Errors..
•
•
•
•
•
The sample:
Glassware / pipettes / balances:
Reagents / calibrators / controls:
The application:
The instrument:
– operational limitations
• temperature control/read times/mixing/carry-over
– lack of maintenance
• worn tubing / optics / cuvettes / probes
Other Factors..
• Calculation errors:
– incorrect factor / wrong calibration values
• Transcription errors:
• Dilutions errors:
– incorrect dilution or dilution factor used
• Lack of training:
• The human factor:
– tiredness / carelessness / stress
Post - Analytical Errors..
• The prompt and correct delivery of the
correct report on the correct patient to
the correct Doctor.
• How the Clinician interprets the data to
the full benefit of the patient.
Accuracy ?
How correct
your result is.
Precision ?
The
reproducibility
of your results.
Accurate and Precise..
Imprecise but Accurate !
Precise but Inaccurate !
Specificity ?
• The ability of a method to measure solely
the component of interest.
• A lack of specificity will affect accuracy
– falsely elevated values
• hormones and drugs
– falsely low values
• BCP method with bovine albumin
Sensitivity ?
• The ability to detect small quantities
of a measured component.
– will affect both precision and accuracy at the
bottom end of the assay range.
Normal Distribution..
Frequency
Mean value (x)
Measured value
Values fall randomly about a mean value.
Precision ?
• How disperse the values are.
• Quantified by measuring the Standard
Deviation (SD) of the set of results.
Standard Deviation (SD)..
SD =
(
(xi - x)
2
)
n -1
The lower the SD the better the
Precision.
Example:
Mean result (x) = 100 mmol/L
Standard deviation (SD) = 1.0 mmol/L
Number of results (n) = 100
Mean +/- 1SD..
Frequency
-1SD
x
+1SD
68%
99 100 101
Values fall randomly about a mean value.
Mean +/- 2SD..
Frequency
-2SD
x
+2SD
95%
98
100
102
Values fall randomly about a mean value.
Which is more Precise ?
Potassium SD = 0.1 mmol/L
Sodium
SD = 2.0 mmol/L
Coefficient of Variation..
CV =
SD
x 100%
Mean (x)
A %CV takes into consideration the
magnitude of the overall result.
Example:
Potassium %CV = (0.1 / 5.0) x 100%
= 2.0%
Sodium %CV = (2.0 / 140) x 100%
= 1.4%
Sodium has the better CV and in this
example is performing better than
potassium.
Interpretation..
10 40
41 50
51 70
71 100
101 120
unacceptable performance
need for improvement
acceptable
good
excellent
TS Calculations
V = (Result - Mean for Comparison) x 100
Mean for Comparison
The mean for comparison could be either:
– the all method mean
– your method mean
– your instrument mean
TS Calculations
TS = Log10 (3.16 x TCV)
x 100
V
TCV is Target Coefficient of Variation
TS Calculations
TS = Log10 (3.16 x TCV)
x 100
V
3.16 is selected as a constant because:
– the log10 of 3.16 is 0.5
– so if V = TCV, then the target score will be 50
TS =
log10 3.16 x TCV x 100
V
=
log10 3.16 x 3.7
3.7
=
log10 (3.16) x 100
=
50
x 100
How can Analytical
Quality be
Controlled ?
• Internal Quality Control (IQC).
– daily monitoring of quality control sera
• External Quality Assessment (EQA).
– comparing of performance to other laboratories.
Internal Quality Control..
• Daily monitoring
– precision
– accuracy
• Quality control sera
– results within control limits indicates
that analytical system is running
satisfactorily
What is Acceptable ?
A sodium control has a target value
of 140 mmol/L
139 mmol/L
120 mmol/L
140 mmol/L
160 mmol/L
141 mmol/L
180 mmol/L
What is Acceptable ?
• A range of acceptable values is established
• Sodium Control:- 137 143mmol/L.
What are the Options ?
• Unassayed serum:
– the cheaper option !
• but the laboratory must establish its own ranges
– cannot be used to assess accuracy !
• no externally assigned target values
• Assayed serum:
– with predetermined targets and ranges
• established by the manufacturer.
Unassayed Serum..
• Analysed extensively by the laboratory.
– a minimum of 20 sets of data generated
– a mean +/- 2SD range established
• 95% of results acceptable
– some laboratories may adopt tighter ranges
Assayed Serum..
• Targets and ranges generated by the
manufacturer:
– abc utilises RIQAS
• database of 5,000 laboratories
• method / instrument / temperature specific values
Levey Jennings Chart
+2SD
143
+1SD
141.5
X
X
X
X
Mean
X
X
X
X
140
X
X
X
X
X
X
-1SD
X
-2SD
X
X
138.5
137
Levey Jennings Chart
+2SD
143
+1SD
141.5
X
Mean
X
X
X
X
X
X
X
-1SD
-2SD
X
X
X
X
X
X
140
X
X
X
138.5
137
Levey Jennings Chart
+2SD
143
+1SD
X
X
X
X
Mean
X
X
X
X
-1SD
-2SD
X
X
X
X
X
141.5
X
X
140
X
X
138.5
137
Levey Jennings Chart
+2SD
143
+1SD
X
X
X
X
X
Mean
X
X
X
X
X
X
X
X
X
X
141.5
X
X
140
-1SD
138.5
-2SD
137
Levey Jennings Chart
+2SD
143
X
X
X
X
+1SD
141.5
X
X
X
Mean
140
X
X
-1SD
-2SD
X
X
138.5
X
X
X
X
X
X
137
Levey Jennings Chart
+2SD
143
X
X
X
+1SD
141.5
X
X
X
Mean
X
X
X
X
X
X
140
X
-1SD
X
-2SD
X
X
X
138.5
137
Levey Jennings Chart
+2SD
143
+1SD
X
X
X
X
141.5
X
X
X
X
X
Mean
140
X
X
-1SD
-2SD
X
X
X
X
X
X
138.5
137
Westgard Rules..
• Decision criteria is dependent on the
precision of the method or analyser
– the less precise the method the more
difficult the decision.
• Westgard provides multiple QC rules:– defines acceptability
• minimises false rejections
• maintains high error detection
Westgard Flowchart..
Control data
No
1 point
In control – report data
outside 2 SD
Yes
1 point
outside 3 SD
No
No
2 consecutive
No
Difference between
4 consecutive control
No
values outside
2 controls within
the same 2 SD
a run
of the mean and
exceeds 4 SD
further than
values on one side
1 SD from the mean
Yes
10 consecutive
No
values
on one side of
the mean
Yes
Yes
Yes
Out of control – reject analytical run
Yes
External Quality
Assessment..
.. the main objective of EQA is not to
bring about day to day consistency
but to establish inter-laboratory
comparability
EQA Options..
• International / National / Regional
• International schemes provide:– a larger database of results
– a wider range of analytical methods
– a global representation of diagnostic
manufacturers
• Compulsory or Voluntary
A Typical EQA Scheme..
• Participants receive unknown samples.
– these are analysed ‘blind’
– the results returned to scheme
organiser
– they are statistically analysed
– to generate a comparative report
– report sent to participant
RIQAS
•
abc
International Quality Assessment Scheme
– launched in 1988
– 5000 participants
• Management tool
– to assess, review and improve performance
RIQAS..
• Annual subscription
– two six monthly cycles
• Weekly samples
– one vial reconstituted per week
– tested blind as if a patient sample
• Results reported back to abc
– statistically analysed
• Weekly Report generated