R p
advertisement

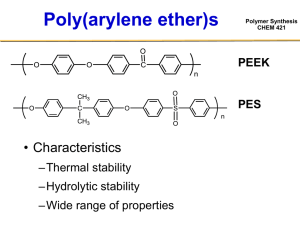
Polymer Synthesis CHEM 421 Free Radical Polymerizations Polymer Synthesis CHEM 421 Reading assignments: Chapter 3-3, 3-4, 3-5, 3-8 (Odian) Free Radical Solution Polymerizations Polymer Synthesis CHEM 421 • Initiation kd I 2 R d [R•] / dt = 2 kd [I] = 2 f kd [I] R + ki M R M - d [R•] / dt = ki [R•] [M] • Propagation R M + nM ki Pn M rp = kp [M•] [M] • Termination Pn + Pm kt Dead Polymer - d [P•] / dt = 2 kt [P•]2 Free Radical Solution Polymerizations • Overall rate expression –Assumptions? Rp = kp [M] [M•] » Steady State Approximation (Ri = Rt) 2 f kd [I] = 2 kt [M•]2 [M•] = (kd f [I] / kt)1/2 Rp = kp [M] (kd f [I] / kt)1/2 Polymer Synthesis CHEM 421 Free Radical Solution Polymerizations • Overall rate expression Rp = kp [M] (kd f [I] / kt)1/2 –Rate is first order in monomer –Rate is ½ order in initiator • To increase Rp Polymer Synthesis CHEM 421 Determination of Orders of Reaction Polymer Synthesis T =75 C, P= 4000 psig, CO2 = 0.74 g/ml CHEM 421 Rp = ([VF2]IN-[VF2]OUT)/ 2 .5 E -0 4 2 .5 E -0 4 [DEPDC]IN =3 mM Rp (mol/L x sec) 2 .0 E -0 4 2 .0 E -0 4 1 .5 E -0 4 1 .5 E -0 4 I* 1 .0 E -0 4 1 .0 E -0 4 5 .0 E -0 5 5 .0 E -0 5 0 .0 E + 0 0 0 .0 E + 0 0 0 0 .5 1 1 .5 2 0 0 .0 1 [VF2]out (mol/L) Rp kp [Iout-I*] 0.5 [VF2]out1.0 0 .0 2 [I]out 0 .0 3 0.5 0 .0 4 (mol/L) 0 .0 5 Experimental Ways of Determining Rp Polymer Synthesis CHEM 421 • Separation and isolation of product –Precipitation –Chromatography • Chemical analysis –Titration of un-reacted double bonds… • Spectroscopic methods –UV/Vis, NMR, FTIR • Refractive index changes • Dilatometry • Calorimetry React-IR Polymer Synthesis CHEM 421 t = 30 s, 32 scans reaction conditions: p = 5000 p.s.i., T = 35 °C, 73 mol-% PDD in feed phase separation emulsion Dilatometer Polymer Synthesis CHEM 421 Free Radical Solution Polymerizations • Overall rate expression Rp = kp [M] (kd f [I] / kt)1/2 –Rate is first order in monomer –Rate is ½ order in initiator • Qualitative picture…? Polymer Synthesis CHEM 421 Free Radical Solution Polymerizations Polymer Synthesis CHEM 421 • Kinetic Chain Length (٧) –Average number of monomer units polymerized per chain initiated Rp Rp Ri Rt ٧= — = — kp [M] [M•] kp [M] kp [M] ٧ = ———— = ——— = ————— 2 (kt kd f [I])1/2 2 kt [M•]2 2 kt [M•] Free Radical Solution Polymerizations • Kinetic Chain Length (٧) kp [M] ٧ = ————— 2 (kt kd f [I])1/2 –To increase molecular weight… –To increase Rate of Polymerization Polymer Synthesis CHEM 421 Kinetic Chain Length (٧) vs Degree of Polymerization (DP) • Termination by combination DP = 2٧ • Termination by disproportionation DP = ٧ Polymer Synthesis CHEM 421 Polymer Synthesis CHEM 421 Classes of Free Radical Initiators Polymer Synthesis CHEM 421 Initiator Decompositions Polymer Synthesis CHEM 421 • Differences in the decomposition rates of various initiators can be expressed in terms of initiator half life • The rate of initiator disappearance is: - d[I] / dt = kd [I] • Integrate: [I] = [I]0 e-k t d [I]0 log —— = kdt [I] or • Half life? [I]0 set: [I] = —— 2 t1/2 0.63 = ——— kd Polymer Synthesis CHEM 421 Factors which Influence Initiator Choice Polymer Synthesis CHEM 421 • Temperature of polymerization • Solubility of the initiator • End group control or functionalization HO OH + Termination by combination HO PBd OH Factors which Influence Initiator Choice Polymer Synthesis CHEM 421 • End group control or functionalization O O K S O O CF2 CF2 CF2 CF CF2 O CF O O O O OCF3 S K CF2CF2CF2CF2Br F3CF2CF2C CF2 CF2 CF2 CF OCF3 CF2 CF CF2CF2CF3 O CF2CF2CF2CF2Br Pulsed-laser Polymerization in Polymer Synthesis Homogeneous Phase: Determination of kp CHEM 421 Pulsed-laser Polymerization in Polymer Synthesis Homogeneous Phase: Determination of kp CHEM 421 Evaluation of kp Polymer Synthesis CHEM 421 Kinetic Chain Length Profile in a PLP Experiment Polymer Synthesis CHEM 421 Li=i kp [M] td Polymer Synthesis CHEM 421 Li=i kp [M] td Polymer Synthesis CHEM 421