Matter_ Mixtures_ Pure Substances
advertisement

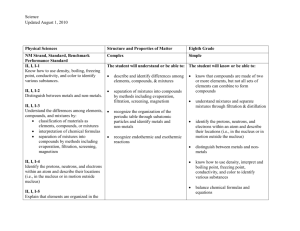
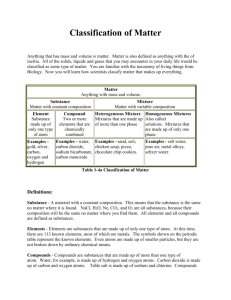
Aim: What is matter? Do Now: A cup of gold colored metal beads was measured to have a mass 425 grams. By water displacement, the volume of the beads was calculated to be 48.0 cm3. Given the following densities, identify the metal. Gold: 19.3 g/mL Copper: 8.86 g/mL Bronze: 9.87 g/mL HOMEWORK: GO ON THE SCHOOL WEBSITE. SELECT “HOMEWORK #1 MATTER AND PROPERTIES”. ON A SEPARATE SHEET OF PAPER RECORD YOUR ANSWER AND EXPLAIN YOUR REASONING FOR YOUR CHOICE. Matter •Matter is anything that has mas mass and volume •Mass and volume are examples of extensive properties •Extensive properties are properties that depend on the amount of matter in a sample •Intensive properties are properties that depend on the type of matter in a sample, not the amount of matter (i.e., density) Physical change vs. Chemical Change PHYSICAL CHANGE •During a physical change, some properties of a material change, but the composition of the material does not change CHEMICAL CHANGE •During a chemical change, the composition of the matter always changes •A new product is not formed •Chemical changes produce new substances with different chemical make-ups and properties than the original substance. •Examples: aluminum foil is cut in half, water evaporates from the surface of the ocean, juice freezes •Examples: milk goes sour, gasoline is ignited, your body digests food, metal rusts •Physical changes can be classified as reversible or irreversible Mixtures •A mixture is a physical blend of two or more components. •Based on the distribution of components, mixtures can be classified as heterogeneous mixtures or as homogeneous mixtures •Heterogeneous mixtures are a mixture in which the composition is not uniform throughout. • Oil and vinegar, chicken noodle soup •Homogeneous mixtures or solutions are mixtures in which the composition is uniform throughout. • oil, vinegar, cup of coffee, salt water Elements and Compounds •An element is the simplest form of matter that has a unique set of properties. •Oxygen, hydrogen •A compound is a substance that contains two or more elements chemically combined in a fixed portion. •Salt, sugar •Compounds can be broken down into simpler substances by chemical means, but elements cannot. -Particles are not uniformly distributed. Particles are physically combined. -Particles are uniformly distributed. Particles are physically combined. -Simplest form of matter. Ex: O2 -Two or more different elements chemically combined. Ex: H2O Sample Problems Which substance can not be decomposed into simpler substances? (1) ammonia (2) aluminum (3)methane (4)methanol Sample Problems cont. Which statement describes a characteristic of all compounds? (1) Compounds contain one element, only. (2) Compounds contain two elements, only. (3) Compounds can be decomposed by chemical means. (4) Compounds can be decomposed by physical means. Sample Problems cont. Which formula represents a binary compound? (1) NH4NO3 (2) CH4 (3) CH3CoCH3 (4) CaCO3 Sample Problems cont. An example of a heterogeneous mixture is: (1) soil (2) sugar (3) carbon monoxide (4) carbon dioxide