MetalsNonmetalsMetalloids
advertisement

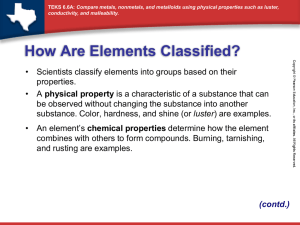
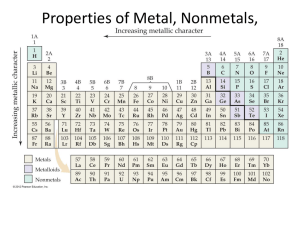
8th grade Physical Science Make sure that you included the following on your graphic organizer Malleable Ductile Luster- shiny High Density Good Conductors of heat and electricity High melting point Hardness Most are solids except for Mercury Corrosive-react water with oxygen and React strongly with acids but not bases Form ionic bonds Tend to react with nonmetals Make sure you included the following in the nonmetals section of your graphic organizer Poor Conductors of heat and electricity Dull Brittle Most solids- (not malleable or ductile) are gases at room temperature Low density Low melting point Hard or soft Various colors Tend to react with metals Form ionic and covalent bonds Make sure you included the following in the metalloid section of your graphic organizer Metalloids have properties of both metals and non-metals. Can be shiny or dull Semiconductors- conduct heat and electricity better than nonmetals but not as good as metals Solid at room temperature Can be ductile or brittle Can be malleable or not Now it is time to color code your copy of the periodic table Take out your periodic table and SHADE all of the metals blue, the nonmetals yellow and the metalloids green Label each of the families of the periodic table. Families are the VERTICAL rows #1-18 Families have similar properties as each other Row #1 are the Alkali metals Row #2 are the Alkaline Earth metals Rows 3-12 are the Transition metals Row 13 is the Boron group Row 14 is the Carbon group Row 15 is the Nitrogen group Row 16 is the Oxygen group Row 17 are the Halogens Row 18 are the Noble gases