AMMONIUM DICHROMATE
advertisement
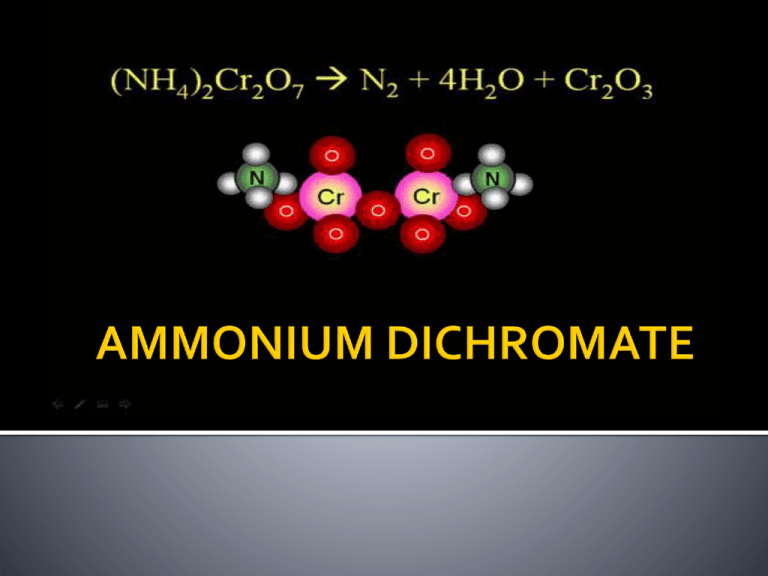
What is Ammonium Dichromate? Properties of (NH4)2Cr2O7 It’s hazards Applications in daily life An experiment with Ammonium Dichromate WHAT IS AMMONIUM DICHROMATE? (NH4)2Cr2O7 A salt that forms orange, monoclinic crystals; made from ammonium sulfate and sodium dichromate; soluble in water and alcohol; ignites readily; used in photography, lithography, pyrotechnics, and dyeing. Also known as ammonium bichromate. Formula: (NH4)2Cr2O7 Molecular Weight: 252.07 g/mol Melting Point: 1800C Boiling Point: Decomposes State at Room Temperature: Solid Molecular or Ionic: Molecular Molecular Geometry: Tetrahedral Discription: Orange Crystals Uses: Oil Purification, Pickling, Leather Tanning, Photography, Wood Preservative, Catalysts Interesting Facts: Used in high school chemistry labs to make "volcanos" Acute Health Effects: Chronic Health Effects: Cancer Hazard: Pyrotechnics Photography Lithography Dyeing pigments Manufacturing of alizarin Leather tanning Oil purification Research Question: What will be the result if ammonium dichromate reacts with oxygen? In my opinion, in the experiment the ammonium dichromate will decomposes and it will be a combustion reaction and as a result of it, there will be some green or grey dust particles. Metal Container 10g Ammonium Dichromate Bunsen Burner http://www.youtube.com/watch?v=CW4hN0dYnkM&list=LPUQJSgA0UH5k&index =1&feature=plcp http://en.wikipedia.org/wiki/Ammonium_dichromate#Saf ety http://www.chemistryland.com/CHM130S/08Equations/TypesReactions/TypesReactions.htm http://www.emsb.qc.ca/laurenhill/science/oxidation.html http://nj.gov/health/eoh/rtkweb/documents/fs/0097.pdf http://www.juliantrubin.com/encyclopedia/earthsciences/ volcano.html http://www.chemistryreference.com/q_compounds.asp?CAS=7789-09-5 http://wiki.answers.com/Q/Describe_the_action_of_heat_ on_ammonium_dichromate THANK YOU FOR LISTENING…