Intermolecular forces

Chemistry 11
Resource: Chang’s Chemistry Chapter 9
Objectives
Predict whether or not a molecule is polar from its molecular shape and bond polarities.
Objectives
Describe the types of intermolecular forces
(attractions between molecules that have temporary dipoles, permanent dipoles or hydrogen bonding) and explain how they arise from the structural features of molecules.
Describe and explain how intermolecular forces affect boiling points of substances.
Polarity
Recall the compound HF.
How did we predict / know that this was a polar molecule?
Demonstration of symbols.
We can also predict the polarity of a molecule based on its geometry.
Polarity
A dipole moment is a quantitative measure of the polarity of a bond.
Molecules without dipole moments are nonpolar.
Molecules with dipole moments are polar.
Does HF have dipole moments? HCl?
H
2
? O
2
?
Polarity
A dipole moment is a quantitative measure of the polarity of a bond.
Molecules without dipole moments are nonpolar.
Molecules with dipole moments are polar.
Does HF have dipole moments? HCl?
H
2
? O
2
?
Polarity
Consider the compound BF
3
.
Based on electronegativity, the B-F bond polar?
Now consider the geometry of BF
3.
Is the molecule (as a whole) polar?
Polarity
The difference in electronegativities of B-F is 2.0, so YES, the bond is polar.
The geometry, however is very symmetrical, so NO, the molecule is not polar.
It is important to consider both the electronegativities of the atoms and geometry of the molecule to determine polarity.
Polarity
Construct the following molecules and label them. Are they polar or not?
HBr
H
2
S
Cl
2
CBr
4
I
2
NO
3
NH
3
C
6
H
6
Kinetic molecular theory
Compare solids, liquids, and gases in terms of volume, shape, density, compressibility, and molecular motion.
State Volume/ shape
Density Compressibility Motion solid liquid gas
Intermolecular forces
What does intermolecular mean?
What does intramolecular mean?
Which one is involved in bonding?
Intermolecular forces
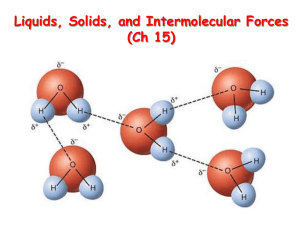
Intermolecular forces are the attractive forces between molecules. Also called van der Waals forces.
Are there intermolecular forces between molecules in:
solids?
liquids?
gases?
Why are intermolecular forces important?
Intermolecular forces
Molecular geometry and intermolecular forces are responsible for the gross properties of matter, such as:
physical appearance
melting point
boiling point
Intermolecular forces
It takes about 41 kJ to vaporize one mole of water and about 930 kJ to break the two O-H bonds in 1 mole of water.
What does this imply about the strengths of inter- and intramolecular forces?
Intermolecular forces
Generally, intermolecular forces are much weaker than intramolecular forces.
Which physical property (physical appearance, boiling point, or melting point) is a better measure of the strength of intermolecular forces?
Intermolecular forces
Boiling point is the best indication of the intermolecular forces in a substance.
Evaporation involves almost completely overcoming the attractive forces between molecules.
If the boiling point is high, what does it tell you about the intermolecular forces?
Intermolecular forces
Types of intermolecular forces:
1.
2.
3.
dipole-dipole dipole-induced dipole
(London) dispersion forces
Intermolecular forces
Attractive forces between polar molecules (molecules with dipole moments).
How do you think the polar molecules would line up?
How would an ion interact with a polar molecule?
What kind of force is responsible?
Dipole-dipole
Intermolecular forces
There is a specific dipole-dipole interaction called the hydrogen bond .
This is a misnomer because it is not actually a bond.
It is the interaction between the hydrogen atom in a polar bond and an electronegative atom.
Dipole-dipole
Intermolecular forces
What would happen if you place an ion near a nonpolar molecule?
Induced dipole - dipole
Intermolecular forces
Although a nonpolar molecule may not possess dipole moments, dipoles can be induced .
Ions and polar molecules can induce dipoles in nonpolar molecules.
The separation of positive and negative charges in a nonpolar molecule is due to the proximity or a polar molecule.
Induced dipole - dipole
Intermolecular forces
Does this mean all substances with ions/polar molecules will have induced dipole – dipole forces?
What factors might affect the likelihood of an induced dipole?
Induced dipole - dipole
Intermolecular forces
Because electrons are always moving, it is possible that a dipole can exist in an atom / nonpolar molecule for an instant.
This is called a temporary dipole.
A temporary dipole can induce dipoles in the surrounding atoms / molecules.
(London) dispersion forces
Intermolecular forces
In gases, these temporary dipoles do not have much impact.
At low temperatures, however, they can cause nonpolar substances to condense.
Why is this so?
(London) dispersion forces
Intermolecular forces
Melting points of similar nonpolar compounds
Compound
CH
4
CF
4
CCl
4
CBr
4
CI
4
Melting point (C)
-182.5
-150.0
-23.0
90.0
171.0
What trend do you notice? What is responsible for this?
(London) dispersion forces
Intermolecular forces
Identify the type of intermolecular forces that exist between:
HBr and H
2
S
Cl
2 and CBr
4
I
2 and NO
3
NH
3 and C
6
H
6
Intermolecular forces
Identify the type of intermolecular forces that exist between:
HBr and H
2
S – dipole-dipole
Cl
2 and CBr
4
– dispersion
I
2 and NO
3
– ion-induced and dispersion
NH
3 and C dispersion
6
H
6
– dipole-induced dipole and
Intermolecular forces
Relationship between intermolecular forces and physical properties
Property Effect of increased intermolecular forces
Melting point
Boiling point
Viscosity
Surface tension
Phase at room temperature
Homework
Water is a very common (and yet very unique) substance on Earth.
What are the properties of water? Explain its properties using what we’ve learned about molecular geometry and intermolecular forces.
Quiz next class on molecular geometry and intermolecular forces.
Long test on Wednesday, 25 February.
Coverage: Atomic theory and bonding