Polarity and Intermolecular Forces
advertisement
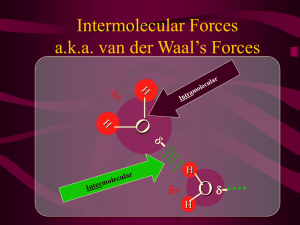
Comparing Acid Strengths by Comparing Structures Look at the stability of the conjugate base. The more stable the conjugate base, the stronger its acid. Electronegativity Size/polarizability Resonance Stabilization Induction Hybrid orbital containing electrons Which H is more acidic? Where does the equilibrium lie? O O + OH Cl Cl Cl O O O - O- + Cl Cl Cl OH Does an Acid-Base Reaction Occur? Write the products. (CH3)3N + NH2- Bond Polarity - Part I A bond is polar when the charge is not equally shared between the two atoms. The more electronegative atom will have a partial negative charge (δ-). The arrow shows the dipole moment. Here we show partial charges. Bond Polarity - Part II A polar bond has a dipole moment μ: μ(in debyes) = 4.8 δ d δ is the charge at either end of the dipole d is the bond length in angstroms (charge separation) (1Å=10-10m) dipole moment, μ bond length, d Bond Polarity - Part II μ(in D) = 4.8 δ d The dipole moment μ gives a quantitative measure of the polarity of a bond. C=O (2.4D) is more polar than C-O (0.86 D) Bond Dipole Moments from Wade, 7th ed (Table 2-1) C-O 0.86D C-N 0.22D C-F 1.51D C=O 2.4D C≡N 3.6D C - Cl 1.56D H-O 1.53D H-N 1.31D C - Br 1.48D H-C 0.3D C-I 1.29D Bond Polarity - Part II μ(in D) = 4.8 δ d Knowing μ and d allows the charge separation δ to be calculated. C=O has a dipole moment of 2.4D and a bond length of 1.21Å. δ = 2.4/(4.8x1.21)= 0.41 C-O has a dipole moment of 0.86D and a bond length of 1.43Å. δ = 0.86/(4.8x1.43)= 0.13 Molecular Polarity The polarity (or dipole moment) of a molecule is the vector sum of the dipole moment for each bond in the molecule. A molecule with a significant dipole moment is polar. A molecule with little or no dipole moment is considered nonpolar. Molecular Polarity The dipole moment of a molecule can be measured. The dipole moments of the individual bonds can then be estimated. Lone pairs contribute to the dipole moments. Intermolecular Forces arise from the charged nature of the subatomic particles (electrons and protons). are responsible for the cohesiveness of materials. are what determine physical properties of pure substances such as melting point, boiling point, vapor pressure, and solubility. Intermolecular Forces Substances that are gases at room temperature have weak intermolecular forces. Substances that are condensed (liquids or solids) at room temperature have much stronger intermolecular forces. If intermolecular forces did not exist, all substances would be gases, even at extremely low temperatures. Intermolecular Forces Dipole-dipole generally attractive Hydrogen bonding a special category of very strong dipole-dipole force that involves the attraction between an electropositive H atom and nonbonding electrons on an electronegative atom (usually N, O, F, or Cl) London dispersion force instantaneous dipole-induced dipole increases with increasing surface area of the molecule present in all molecules Intermolecular Forces Which will have the higher boiling point? or Intermolecular Forces Why does CCl4 have the higher boiling point? chloroform, CHCl3 (μ = 1.0D) or carbon tetrachloride, CCl4 (μ = 0) bp CHCl3 = 62°C bp CCl4 = 77°C Intermolecular Forces and Solubility “Like dissolves like.” Polar substances dissolve in polar solvents. Nonpolar substances dissolve in nonpolar solvents. The other pairings (polar substance/nonpolar solvent and nonpolar substance / polar solvent) will not dissolve. Intermolecular Forces and Solubility For one substance to dissolve in another, there must be an attraction similar in magnitude to the forces holding the solvent together. In water, H bonding holds the molecules of water together pretty tightly. For a substance to dissolve in water, there must be an attraction between the substance and water that is close in magnitude to those H bonds. Ions, alcohols, and ethers all dissolve in water…can you show why? Intermolecular Forces and Solubility Carbon tetrachloride does NOT dissolve in water. Water is held together by H bonds, a strong intermolecular interaction. Carbon tetrachloride is nonpolar. The only force of attraction between CCl4 and H2O is dispersion, and that is not strong enough to push apart the Hbonded water molecules. Intermolecular Forces Which are soluble in water and why? Phosphatidyl choline – a lipid found in cell membranes http://www.agen.ufl.edu/~chyn/age2062/lect/lect_06/4_18.GIF Intermolecular Forces and the Cell Membrane http://www.youtube.com/watch?v=UL R79TiUj80&feature=related