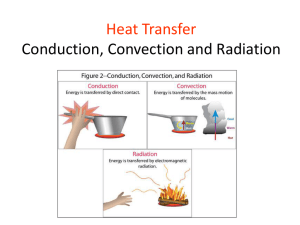
Reactions
advertisement

Reactions B. Seven Diatomic Elements • (Super Seven) never appear alone in nature. They are chemically combined in compounds or with themselves. These include hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine. • H2, N2, O2, F2, Cl2, Br2, I2 IV. Types of Chemical Reactions A. Combination or Synthesis 1. A reaction in which two or more substance combine to form a single product 2. Examples: 2. Examples: • • • • • 2H2 + O2 2 H2O 4Al + 3O2 2Al2O3 Ca + Cl2 CaCl2 2KCl + 3O2 2KClO3 Notice that in each example, a compound is formed from elements or smaller compounds. This may be represented by the following general equation: A + B AB B. Decomposition Reaction 1. A reaction in which a compound is broken down into simpler substances such as elements or smaller compounds 2. Examples 2. Examples • 2HgO • 2H2O ∆ 2Hg + O2 𝑒𝑙𝑒𝑐𝑡𝑟𝑐𝑖𝑡𝑦 𝐶𝑎𝑡𝑎𝑙𝑦𝑠𝑡 2H2 + O2 • 2 KClO3 2KCl + 3O2 • Decomposition reactions are generally the opposite of composition reactions. Such a reaction may be represented by the following general equation: AB A + B C. Single Replacement Reaction 1. A reaction in which one element reacts with a compound to form a different element and another compound 2. Examples 2. Examples • • • • Cu + 2AgNO3 Ag + Cu(NO3)2 Zn + Cu(NO3)2 Cu + Zn(NO3)2 Mg + 2HCl H2 + ZnCl2 These reactions occur according to the activity series of metals. Elements on the top of the list may replace elements below them in a chemical reaction. For the Halogens, elements may replace those below them in the group. • The general equation for a single replacement reactions looks like: A + BC B + AC D. Double Replacement Reactions 1. A reaction in which the metals (or cations) present in two compounds changes places to form two new compounds. 2. Examples 2. Examples • AgNO3 + NaCl AgCl + NaNO3 • NaOH + HCl NaCl + H2O • Ba(NO3)2 + Na2SO4 BaSO4 + 2NaNO3 • The general equation for a double replacement reactions is: AB + CD AD + CB • These reactions often use the solubility rules to predict precipitate formation. E. Combustion Reaction 1. A reaction in which oxygen reacts with another substance usually producing heat and light. (We will be dealing primarily with the combustion of hydrocarbon fuels) 2. There are two kinds of combustions: complete combustion and incomplete combustion 3. Examples • CxHy + O2 CO2 + H2O • CxHy + O2 CO + C + H2O 4. Elemental carbon or soot, among other things may be formed during incomplete combustion. F. Oxidation – Reduction Reactions 1. A reaction in which the oxidation number of an element changes. 2. Think of a number line. If the oxidation number moves to the right on the number line (becomes less negative or more positive) the element is oxidized. If the oxidation number moves to the left on the number line (becomes more negative or less positive) the element is reduced. 3. To calculate the oxidation of the element, use the charge of the element or polyatomic ion to determine the charge of the metal in each substance. Elements in their natural state have an oxidation number of zero. 4. Examples • Ag + NaCl AgCl + Na • Al + Cu(NO3)2 Cu + Al(NO3)3 5. For this course, we will concentrate on oxidation-reduction reactions that are also single replacement reactions, but others do exist. G. What type of chemical reaction is represented by the following equations? • SR • Ca + BaO Ba + CaO • CB • 2C3H6 + 9O2 6CO2 + 6H2O • S • Ca + O2 CaO • DR • Na3PO4 + BaCl2 Ba3(PO4)2 + NaCl • D • MgCl2 Mg + Cl2 H. Nuclear Reactions 1. A reaction in which nucleotides (particles) react during nuclear fusion or fission. 2. Fusion – is the combination of two smaller nucleotides for form a larger, more massive particle. This is what happens in our sun. 3. Fission – is the splitting of a large, unstable nucleotide into smaller particles. This occurs in nuclear reactors. 4.The particles involved include the following • Alpha particles, a, look like helium ions: 𝟒𝟐𝑯𝒆𝟐+ • Beta particles, b, look like electrons: −𝟏𝟎𝒆 • Positrons look like positive beta particles: +𝟏𝟎𝒆 • Neutrons are just neutrons: 𝟏𝟎𝒏 • Protons are often written as hydrogen ions: 𝟏𝟏𝑯+ • Gamma Rays – really a form of radiation that accompanies many nuclear reactions: 00𝛾 or just g 5. To balance a nuclear reaction, make sure the total mass numbers on each side of the reaction are equal and the total atomic numbers on each side are equal. 6. Examples 6 4 2 • 3Li + 1H → 2He + 𝟒𝟐𝐇𝐞 6+2=4+x x=4 3+1=2+y y=2 • 14 6𝐶 • 238 92𝑈 • 234 + → 1 1𝐻 234 𝑇ℎ → → 1 0𝑛 + 𝟏𝟒 _____ 𝟕𝐍 𝑇ℎ + _____ 234 𝑃𝑎 + _____ 7. Radiation • Alpha radiation is the least penetrating. It can be stopped by a piece of paper. This is because of the size of the particles. • Beta radiation is moderately penetrating. Aluminum foil is thick enough to stop beta radiation. • Gamma radiation is the most penetrating because it is a form of electromagnetic radiation. Layers of concrete and lead are needed to stop gamma radiation.