IMF: Part 2
advertisement
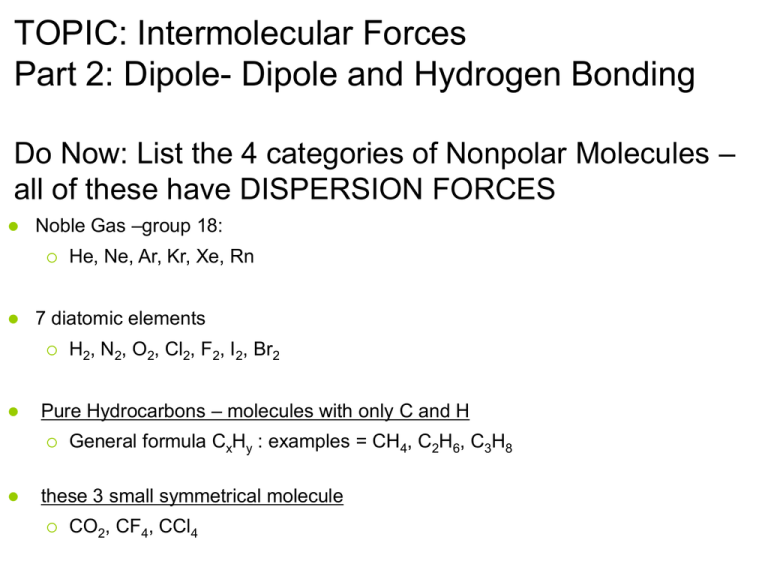
TOPIC: Intermolecular Forces Part 2: Dipole- Dipole and Hydrogen Bonding Do Now: List the 4 categories of Nonpolar Molecules – all of these have DISPERSION FORCES Noble Gas –group 18: 7 diatomic elements H2, N2, O2, Cl2, F2, I2, Br2 Pure Hydrocarbons – molecules with only C and H He, Ne, Ar, Kr, Xe, Rn General formula CxHy : examples = CH4, C2H6, C3H8 these 3 small symmetrical molecule CO2, CF4, CCl4 All molecules have Dispersion forces (the regents calls these Van der Waals) 2 other types of forces (IMF): 1. Dipole-Dipole forces 2. Hydrogen bonds -if one of these are present, they are more important. 2. • • • Dipole-dipole forces: Stronger then dispersion forces occur between polar (asymmetrical) molecules (they have a partial charge at each pole – one is typically much larger than the other) Click here for animation (slide 3 of 13) Dipole-dipole Forces & Polar Molecules Polar Molecule shows permanent separation of charge; has poles: one end partially (-) & one end partially (+); Asymmetrical 3. • • Hydrogen bonds: strongest IMF occur between molecules that have an : H-F H-O or H-N bonds ONLY Strongest Intermolecular Force Hydrogen Bonding Dipole-Dipole Dispersion Hydrogen Bonding H-O N-H Occurs between molecules with H-F, H-O, or H-N bonds Hydrogen Bonding Hydrogen bonding is extreme case of dipole-dipole bonding F, O, and N are all small and electronegative strong electrons attraction H has only 1 electron, so if being pulled away H proton is almost “naked” H end is always positive & F, O, or N end is always negative Strength of Hydrogen Bonding Fluorine most electronegative element, so H-F bonds are most polar and exhibit strongest hydrogen bonding, so strongest IMF H-F is stronger than H-O which is stronger than H-N (H-bonding…sound like FON to me!!!) H H O H O H H-Bonding = strongest IMF much harder to “pull” molecules apart H H C H H H H C H H Dispersion Forces= weakest IMF much easier to “pull” molecules apart Hydrogen bonding: • strongest IMF • influences physical props a great deal H-F > H-O > H-N IMF vs Physical Properties If IMF then: point Melting point Heat of Fusion Change from solid to liquid w/o changing temp Heat of Vaporization Change from liquid to gas w/o changing temp Boiling while: Evaporation Rate Rate at which conc. will go from liquid to gas Why do some substances exist as gases, some as liquids, and some as solids at room temp? #1 reason = IMF If IMF are strong, substance will be solid or liquid at room temp Particles want to clump together If IMF are weak, substance will be gas at room temp Particles free to spread apart Why do some substances exist as gases, some as liquids, and some as solids at room temp? #1 reason = IMF #2 reason = temperature (avg. KE) Temp = average KE If we change T we change KE Increase KE will help “pull” molecules apart (overcome IMF) Indicate type of IMF for each molecule: NH3 • Ar • N2 • HCl • HF • Ne • O2 • HBr • CH3NH2 • Hydrogen bonding Dispersion forces Dispersion forces Dipole-dipole forces Hydrogen bonding Dispersion Dispersion Dipole-dipole Hydrogen bonding