Whiteboard Practice - Belle Vernon Area School District
advertisement

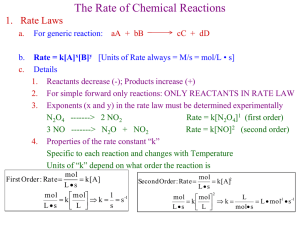
Solutions Review Play as a slideshow! Calculate the molarity of a 0.175 L sugar solution that was prepared with 0.15 moles of sugar? • M = mol/L • M = 0.15 mol/0.175 L • M = 0.86 M Solutions are ________________ mixtures made up of very small particles. • Homogeneous Determine the volume of 0.235 M sugar solution that can be prepared with 0.470 moles of sugar. • M = mol/L • 0.235 M = 0.470 mol/ x L ***cross-multiply • 0.235 x = 0.470 • X = 2.00 L Explain why solutions are classified as mixtures instead of compounds. • The two parts, solute and solvent, are only physically combined, not chemically combined. Also, you can easily separate them with a physical change, such as evaporating off the solvent. Finally, they can combine in different ratios, they do not have a fixed ratio like compounds (example, water is always 2 H and one O, but salt water can have varying percent of salt in it). A salt solution is to be added to a marine aquarium. Calculate the molarity of a salt solution that is prepared by adding water to 18.65 g of NaCl to give a final volume of 250.0 ml. • M = mol/L • 18.65 g x 1 mol/ 58.44 g = 0.3191 mol • M = 0.3191 mol/0.2500 L • M = 1.277 M Pure gold is 24 carat. 14-carat gold contains 14 parts gold and 10 parts other metals. 14-carat gold is said to be a(n) ___________, which is a alloy type of solution. An example of a gaseous air solution is ________________, which is made oxygen up mostly of ______________ and nitrogen when dry. The most common solutions are ______________ solutions. aqueous Calculate the volume of a 3.15 M NaOH (aq) solution that should be used to prepare 250. ml of 0.150 M NaOH (aq). • M1V1 = M2V2 • 3.15 M x V1 = 0.150 M x 250. mL • V1 = 11.9 mL Because of the _______________ _______________, you can see the light beams from car headlights in a fog. • Tyndall Effect You have 3.0 L of 3.0 M HCl. What volume of 2.0 M HCl can you make? • M1V1 = M2V2 • 3.0 M x 3.0 L = 2.0 M x V2 • V2 = 4.5 L 3.5 L of solvent was added to 2.0 L of a 0.88 M solution. What is the new molarity of the solution? • M1V1 = M2V2 • 0.88 M x 2.0 L = M2 x 5.5 L • M2 = 0.32 M Multiple choice: To increase the rate of solution of a solid in water, • a. increase the pressure over the water. • b. decrease the pressure over the water. • c. crush the particles of the solid. • d. chill the water. You need 450 mL of 0.15 M NaOH. All you have available is a 2.0 M stock solution of NaOH. What volume of solvent will you add to the volume required of the stock solution to make your dilution? • M1V1 = M2V2 • 0.15 M x 450 mL = 2.0 M x V2 • V2 = 33.75 mL stock solution • 450 – 33.75 = 416.25 mL solvent • If 15 grams of iodine are dissolved in 1000 mL of alcohol, the alcohol is the (solute, solvent) and the solution is said to be a(n) tincture _______________. • A substance that dissolves other materials is a (solute, solvent). The substance being dissolved is a (solute, solvent). You have 6.0 L of 5.0 M NaCl stock solution. You take 800 mL of that stock solution, and add 500 mL of solvent. -How many moles of NaCl would be present in the new solution? -What is the molarity of new solution? • M = mol/L • 5.0 M = x mol/ 0.800 L • X = 4.0 mol • M1V1 = M2 V 2 • 5.0 M x 800 mL = M2 x 1300 mL • M2 = 3.08 M • In (solutions, suspensions) the substances separate after standing a while. The substances (can also, can not) be separated by filtration. Find the volume of a 0.75 M solution if it contains 39 grams of KOH. • 39 g x 1 mol/56.1 g = 0.695 mol • M = mol/L • 0.75 M = 0.695 mol/x L ***cross-multiply*** • X = 0.927 L • Smoke is an example of a _____________ of solid dirt and dust particles in air. • colloid How many grams of hydrochloric acid (HCl) are present in 3.0 L of a 0.750 M solution? • M = mol/L • 0.750 M = x mol/ 3.0 L • X = 2.25 mol • 2.25 mol x 36.45 g/ 1 mol = 82.0 g • A(n) ________________’s particles are between those of a solution and a suspension. • colloid 110.0 mL of 3.00 M sulfuric acid has 25.0 mL of water added to it. What is the resulting concentration of the solution ? • M1V1 = M2V2 • 3.00 M x 110.0 mL = M2 x 135.0 mL • M2 = 2.44 M • How does a solution behave differently from a suspension when a beam of light is shined through it? • A beam of light will go right through a solution and you will not see it. A suspension exhibits the Tyndall Effect where you will be able to see the beam of light. Acetic acid is purchased as a solution in 17.0 M concentrations. Explain how you would prepare 500.0 mL of a 5.00 M solution. • M1V1 = M2V2 • 17 M x V1 = 5.00 M x 500.0 mL • V1 = 147.1 mL • Put 147.1 mL into a 500 mL volumetric flask. Then add water until the solution is 500 mL total (to the line). • What is the solute in a brass alloy containing 75% copper and 25% zinc? • Zinc (it has a smaller percentage) • Substances that conduct electricity when electrolytes dissolved are said to be ______________, while substances that do NOT conduct electricity when dissolved are said to be Non-electrolytes __________________.