3_percentage_composition
advertisement

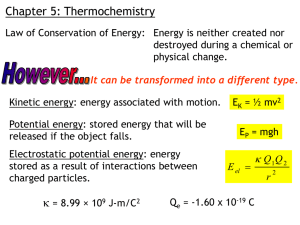
Percentage Composition Try this: What percentage of a car’s mass is made up by the wheels? What information do we need? mass of car body = 1920 kg (without wheels) mass of each wheel = 20 kg (ignore the spare) Percent by mass of wheels . . . % mass wheels = total mass four wheels total mass car (incl wheels) = 4 wheels(20 kg/wheel) [1920 + 4(20)] kg = 80 kg 2000 kg = 0.040 or 4.0% Let’s apply this to Chemistry Calculate the % by mass of C in CO2. Can work either per mole or per molecule. %C per mole CO2 = 12g Carbon [12g C + 2(16g O)] = 12/44 = 27% C in CO2 (to 2 sf) Consider benzene, C6H6 Benzene has 6 C atoms and 6 H atoms. Why is benzene not 50% H by mass? Each H atom is 1.01 u; C atom is 12.01 u. [But, benzene is 50% H in terms of # of atoms.] Calculate % by mass C in C6H6. %C = mass 6 C/mass C6H6 = 6(12.01)/[6(12.01) + 6(1.01)] = 72.06/78.12 = 92.24 % C (to 4 sf) Law of Definite Proportions states that the elements in a chemical compound are always present in the same proportions by mass. or water is water is water. What’s the formula of water found on Mars? H2O. Same as here. The LDP and how not to get ripped off at the drug store . . . The chemical name for Vitamin C is ascorbic acid. “Life” brand vit C: $4.95/100 tablets (100 mg) “Zest for Life” brand natural source vitamin C: $15.95/100 tablets (100 mg) Which is better? (Both are “USP” on label.) Both are ascorbic acid; get the cheapest. Sample Problem A certain compound contains C, H, O. A 650.0 mg sample of the compound contains 257.0 mg C and 50.4 mg H. Find the mass % of O in the compound. mass O = (650.0 – 257.0 – 50.4) mg = 342.6 mg %O = (342.6 mg/650.0 mg) * 100% %O = 52.71 % Similarly for C and H. Now try this one: What mass of Chromium is contained in 54.3 g of potassium dichromate, K2Cr2O7? (mm = molar mass) mass Cr = [mm 2Cr/mm K2Cr2O7] * 54.3 g = [2(52.0)/[2(39.1)+2(52.0)+7(16.0)] * 54.3 g = [104.0/294.2] * 54.3 g = [0.354] * 54.3 g and so mass Cr = 19.2 g in 54.3 g K2Cr2O7. What does the 0.354 represent? 35.4 % by mass Cr in K2Cr2O7.. Challenge (cf p 267 #13) a) Washing soda has the formula NapX1Or, where p and r are unknown. The molar mass of washing soda is 105.99 g/mol. If washing soda is 43.4% Na and 45.3% O by mass, what is the element X? Solution Na: 0.434 * 105.99 g/mol = 46.00 g Na/mol wash soda /23.00 g Na/mol Na = 2 mol Na/mol wash soda O: 0.453 * 105.99 g/mol = 47.97 g O/mol /16.00 g O/mol = 3 mol O/mol wash soda So far we have Na2X1O3 = 105.99 g/mol or 105.99 g – 2(23.00 g) – 3(16.00 g) Na molar mass O molar mass = 11.99 or 12 which is the molar mass of C and so washing soda has the formula Na2CO3. Another Challenge (cf p 267 #14) Consider a soap molecule with the formula C18HxOy(alkali metal)1. If the soap is 70.5% C; 11.5% H; 10.4% O by mass, identify the alkali metal. Solution 1 18 Cs * 12.01 g/mol = 216.18 g /0.705 molar mass of soap = 306.6 g/mol Homework p 260 1 – 10 (try a few) p 262 1 – 6 (do ‘em all) p 264 11 – 20 (try a few) p 266 21 – 30 (try a few) p 267 1, 2, 13, 14 (do these at a minimum)