Protein Misfolding Can Have Deadly Consequences
advertisement

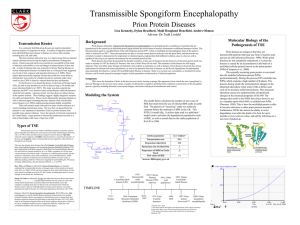
Protein Misfolding Can Have Deadly Consequences Yu Tiantian Li Yihan In April 1996 a paper was published in the medical journal Lancet that generated widespread alarm in the populations of Europe. The paper described a study of 10 persons afflicted with Creutzfeldt-Jakob disease (CJD), a rare, fatal disorder that attacks the brain, causing a loss of motor coordination and dementia. CJD Creutzfeldt–Jakob disease or CJD is a degenerative neurological disorder (brain disease) that is incurable and invariably fatal.The disease is at times called a human form of Mad Cow disease given the fact that Bovine spongiform encephalopathy is the cause of variant Creutzfeldt-Jakob disease in humans. This means that the brain develops holes and takes on a sponge-like texture. Like numerous other diseases, CJD can occur as an inherited disease that runs in certain families. Unlike virtually every other inheritable disease CJD can also be acquired. Until recently, persons who had acquired CJD had been recipients of organs or organ products that were donated by a person with undiagnosed CJD. ??? A disease that runs in families can invariably be traced to a faulty gene, whereas diseases that are acquired from a contaminated source can be traced to an infectious agent. How can the same disease be both inherited and infectious? Kuru Gajdusek showed that these islanders were contracting a fatal neurodegenerative disease— which they called “kuru”—during a funeral ritual in which they ate the brain tissue of a recently deceased relative. Autopsies of the brains of patients who had died of kuru showed a distinct pathology, referred to as spongiform encephalopathy, in which certain brain regions were riddled with microscopic holes (vacuolations), causing the tissue to resemble a sponge. It was soon shown that the brains of islanders suffering from kuru were strikingly similar in microscopic appearance to the brains of persons afflicted with CJD. Infection Kuru Daniel Carleton Gajdusek Daniel Carleton Gajdusek (1923–2008) was an American physician and medical researcher who was the co-recipient (with Baruch S. Blumberg) of the Nobel Prize in Physiology or Medicine in 1976 for work on kuru, the first human prion disease demonstrated to be infectious. Infection In 1968, Gajdusek showed that when extracts prepared from a biopsy of the brain of a person who had died from CJD were injected into a suitable lab animal, that animal did indeed develop a spongiform encephalopathy similar to that of kuru or CJD. Clearly, the extracts contained an infectious agent. Stanley Ben Prusiner Stanley Ben Prusiner (1942- ) is an American neurologist and biochemist. Prusiner discovered prions, He received the Albert Lasker Award for Basic Medical Research in 1994 and the Nobel Prize in Physiology or Medicine in 1997 for his prion research. Prion A prion is an infectious agent composed of protein in a misfolded form. This is in contrast to all other known infectious agents, which must contain nucleic acids (either DNA, RNA, or both) along with protein components. Unlike viruses, the prion that infectious agent responsible for CJD lacked nucleic acid and instead was composed solely of protein. PRPN The prion protein was soon shown to be encoded by a gene (called PRNP) within the cell’s own chromosomes. The gene is expressed within normal brain tissue and encodes a protein designated PrPC (standing for prion protein cellular) that resides at the surface of nerve cells. The precise function of PrPC remains a mystery. A modified version of the protein(PrPSc)is present in the brains of humans with CJD. Unlike the normal PrPC, PrPSc accumulates within nerve cells, forming aggregates that kill the cells. PrPc Vs. PrPSc PrPc PrPSc molecular monomeric fibrils Soluble or not soluble insoluble sensitivity insensitive sensitive Stability of protein stable unstable Tertiary structure α-helical About 45% βsheet Pathogenicity pathogenic Not pathogenic An abnormal prion molecule (PrPSc) can bind to a normal protein molecule (PrPC) and cause the normal protein to fold into the abnormal form. This conversion can be shown to occur in the test tube: addition of PrPSc to a preparation of PrPC can convert the PrPC molecules into the PrPSc conformation. CJD’s acquire Inherited Infection Surgical instruments All this situation is started a chain reaction in which normal protein molecules in the cells are gradually converted to the abnormal prion form. Alzheimer’s disease (AD) Alzheimer’s disease (AD) 10 percent of individuals who are at least 65 years of age 40 percent of individuals who are 80 years or older Alzheimer’s disease (AD) Clinical symptoms: memory loss confusion a loss of reasoning repeat language meaningless repetitive movements Comparison of AD and CJD Similarities: fatal neurodegenerative diseases contain fibrillar deposits of an insoluble material Comparison of AD and CJD Differences: The proteins that form the disease-causing aggregates are unrelated. The parts of the brain that are affected are distinct. The protein responsible for AD is not an infectious agent. Causes of AD AD is caused by the production of a molecule, called the amyloid β-peptide (Aβ) Aβ is originally part of a larger protein called the amyloid precursor protein (APP), which spans the nerve cell membrane. The Aβ peptide is released from the APP molecule following cleavage by two specific enzymes,β-secretase and γ-secretase. The types of the Aβ peptide The one species has a length of 40 amino acids (designated as Aβ40). Another species is with two additional hydrophobic residues (designated as Aβ42) It is the misfolded Aβ42 version of the molecule that has the greatest potential to cause damage to the brain. Overproduction of Aβ42 can be caused by mutations in the APP gene or in genes (PS1,PS2) that encode subunits of γ-secretase. Normal Alzheimer’s Experiment on animal model Animal model: the genetically engineered mice Basis of experiment Injecting the animals with the very same substance that causes the problem, the aggregated Aβ42 peptide. Preclinical test Phase I clinical trial Carried out on a small number of subjects and designed to monitor the safety of the procedure. Outcomes No ill-effects from the injection of the amyloid peptide Preclinical test Phase II clinical trial A large group of subjects and designed to obtain a measure of the effectiveness of the procedure. Outcomes Discontinued because of the serious side effect. However, 30 of the patients continued to be tested from the study provided dramatic evidence that the vaccine had had a major impact on slowing disease progression. Preclinical test Phase III clinical trial Employ large numbers of subjects and compares the effectiveness of the new treatment with standard approaches. Outcomes No outcomes. Other therapeutic strategies Nonsteroidal anti-inflammatory medications Cholestero-lowering medications called statins Have a markedly reduced incidence of Alzheimer’s disease. Have already approved for human use and have been taken by tens of millions of people.