ppt
advertisement

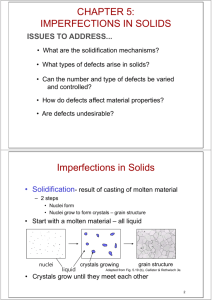
IMPERFECTIONS IN SOLIDS Week 3 1 Imperfections in Solids • Solidification- result of casting of molten material – 2 steps • Nuclei form • Nuclei grow to form crystals – grain structure • Start with a molten material – all liquid nuclei liquid crystals growing grain structure • Crystals grow until they meet each other 2 Polycrystalline Materials Grain Boundaries • regions between crystals • transition from lattice of one region to that of the other • slightly disordered • low density in grain boundaries – high mobility – high diffusivity – high chemical reactivity 3 Solidification Grains can be - equiaxed (roughly same size in all directions) - columnar (elongated grains) ~ 8 cm heat flow Columnar in area with less undercooling Shell of equiaxed grains due to rapid cooling (greater T) near wall Grain Refiner - added to make smaller, more uniform, equiaxed grains. 4 Imperfections in Solids There is no such thing as a perfect crystal. • What are these imperfections? • Why are they important? Many of the important properties of materials are due to the presence of imperfections. Crystalline defect -> a lattice irregularity having one or more of its dimensions on the order of an atomic diameter 5 Types of Imperfections • Vacancy atoms • Interstitial atoms • Substitutional atoms Point defects • Dislocations Line defects • Grain Boundaries Area defects 6 • Vacancies: Point Defects -vacant atomic sites in a structure. Vacancy distortion of planes • Self-Interstitials: -"extra" atoms positioned between atomic sites. selfinterstitial distortion of planes 7 Equilibrium Concentration: Point Defects • Equilibrium concentration varies with temperature! No. of defects No. of potential defect sites. Activation energy -Q Nv = exp v kT N Temperature Boltzmann's constant (1.38 x 10 -23 J/atom-K) (8.62 x 10 -5 eV/atom-K) Each lattice site is a potential vacancy site 8 Measuring Activation Energy • We can get Qv from an experiment. -Q Nv v exp = kT N • Measure this... • Replot it... Nv ln N Nv N slope -Qv /k exponential dependence! T 1/T defect concentration 9 EXAMPLE PROBLEM 4.1 Calculate the equilibrium number of vacancies per cubic meter for copper at 1000C. The energy for vacancy formation is 0.9 eV/atom; the atomic weight and density (at 1000C) for copper are 63.5g/mol and 8.4g/cm3, respectively 10 Estimating Vacancy Concentration Find the equil. # of vacancies in 1m3 of Cu at 1000C. • Given: r = 8.4 g /cm 3 A Cu = 63.5 g/mol Qv = 0.9 eV/atom NA = 6.02 x 1023 atoms/mol -Q Nv = v exp kT N r=N.Acu/V.NA For 1 m3 , N= r x NA A Cu 0.9 eV/atom = 2.7 x 10-4 1273K 8.62 x 10-5 eV/atom-K x 1 m3 = 8.0 x 1028 sites • Answer: Nv = (2.7 x 10-4)(8.0 x 1028) sites = 2.2 x 1025 vacancies 11 Observing Equilibrium Vacancy Concentration. • Low energy electron microscope view of a (110) surface of NiAl. • Increasing T causes surface island of atoms to grow. • Why? The equil. vacancy conc. increases via atom motion from the crystal to the surface, where they join the island. Island grows/shrinks to maintain equil. vancancy conc. in the bulk. 12 IMPURITIES IN SOLIDS • Impurity or foreign atoms will always be present, and some will exist as crystalline point defects • Alloys -> impurity atoms have been added intentionally to impart specific characteristics to the material • Alloying with copper significantly enhances the mechanical strength without depreciating the corrosion resistance appreciably • The addition of impurity atoms to a metal will result in the formation of a solid solution 13 Point Defects in Alloys Two outcomes if impurity (B) added to host (A): • Solid solution of B in A (i.e., random distribution of point defects) OR Substitutional solid soln. (e.g., Cu in Ni) Interstitial solid soln. (e.g., C in Fe) • Solid solution of B in A plus particles of a new phase (usually for a larger amount of B) Second phase particle --different composition --often different structure. 14 Imperfections in Solids Conditions for substitutional solid solution (S.S.) • W. Hume – Rothery rule – 1. r (atomic radius) < 15% – 2. Proximity in periodic table • i.e., similar electronegativities – 3. Same crystal structure for pure metals – 4. Valency • All else being equal, a metal will have a greater tendency to dissolve a metal of higher valency than one of lower valency 15 Substitutional Solid Solution – Cu-Ni • The atomic radii for copper and nickel are 0.128 and 0.125nm, respectively • Both have the FCC crystal structure • Their electronegativities are 1.9 and 1.8 • Valencies for Cu and Ni are +2 16 Imperfections in Solids Application of Hume–Rothery rules – Solid Solutions Element Atomic Crystal ElectroRadius Structure (nm) 1. Would you predict more Al or Ag to dissolve in Zn? 2. More Zn or Al in Cu? Cu C H O Ag Al Co Cr Fe Ni Pd Zn 0.1278 0.071 0.046 0.060 0.1445 0.1431 0.1253 0.1249 0.1241 0.1246 0.1376 0.1332 Valence negativity FCC 1.9 +2 FCC FCC HCP BCC BCC FCC FCC HCP 1.9 1.5 1.8 1.6 1.8 1.8 2.2 1.6 +1 +3 +2 +3 +2 +2 +2 +2 17 Conditions for Interstitial Impurity • The atomic diameter of an interstitial impurity must be substantially smaller than that of the host atoms • The maximum allowable concentration of interstitial impurity atoms is low • Even very small impurity atoms are ordinarily larger than the interstitial sites, and as a consequence they introduce some lattice strains on the adjacent host atoms • Different crystal structures can fill interstitials 18 Specification of Composition – weight percent m1 C1 = x 100 m1 m2 m1 = mass of component 1 – atom percent nm1 C = x 100 nm1 nm 2 ' 1 nm1 = number of moles of component 1 19 Dislocations - Line Defects Dislocations: • are line defects, • slip between crystal planes result when dislocations move, • produce permanent (plastic) deformation. Schematic of Zinc (HCP): • before deformation • after tensile elongation slip steps 20 Imperfections in Solids Linear Defects (Dislocations) – Are one-dimensional defects around which atoms are misaligned Burgers vector (b) represents the magnitude and direction of the distortion of dislocation in a crystal lattice Dislocation Line -> A curve running along the center of a dislocation. • Edge dislocation: – extra half-plane of atoms inserted in a crystal structure – b to dislocation line • Screw dislocation: – spiral planar ramp resulting from shear deformation – b to dislocation line 21 Imperfections in Solids Edge Dislocation 22 Motion of Edge Dislocation • Dislocation motion requires the successive bumping of a half plane of atoms (from left to right here). • Bonds across the slipping planes are broken and remade in succession. Atomic view of edge dislocation motion from left to right as a crystal is sheared. 23 Imperfections in Solids Screw Dislocation Screw Dislocation b Dislocation line Burgers vector b (b) (a) 24 Edge, Screw, and Mixed Dislocations Mixed Edge Screw 25 Imperfections in Solids Dislocations are visible in electron micrographs 51,450 magnified 26 Planar Defects in Solids • One case is a twin boundary (plane) – Essentially a reflection of atom positions across the twin plane. • A twin boundary is a special type of grain boundary across which there is a specific mirror lattice symmetry • Annealing twins are typically found in metals that have the FCC crystal structure, while mechanical twins are observed in BCC and HCP metals 27 Planar Defects in Solids • Stack Fault are found in FCC metals when there is an interruption in the ABCABCABC . . . stacking sequence of close-packed planes • Phase boundaries exist in multiphase materials across which there is a sudden change in physical and/or chemical characteristics 28 Bulk or Volume Defects • Other defects exist in all solid materials that are much larger than those discussed • These include pores, cracks, foreign inclusions, and other phases • They are normally introduced during processing and fabrication steps. 29 Numerical Problems • Problems 4.1 to 4.5 and 4.7 to 4.25 30








![Crystal Imperfections in Solids [7]](http://s3.studylib.net/store/data/008311432_1-73ab0b6bbff880ea6e4c24d3e014fbc2-300x300.png)